Abstract
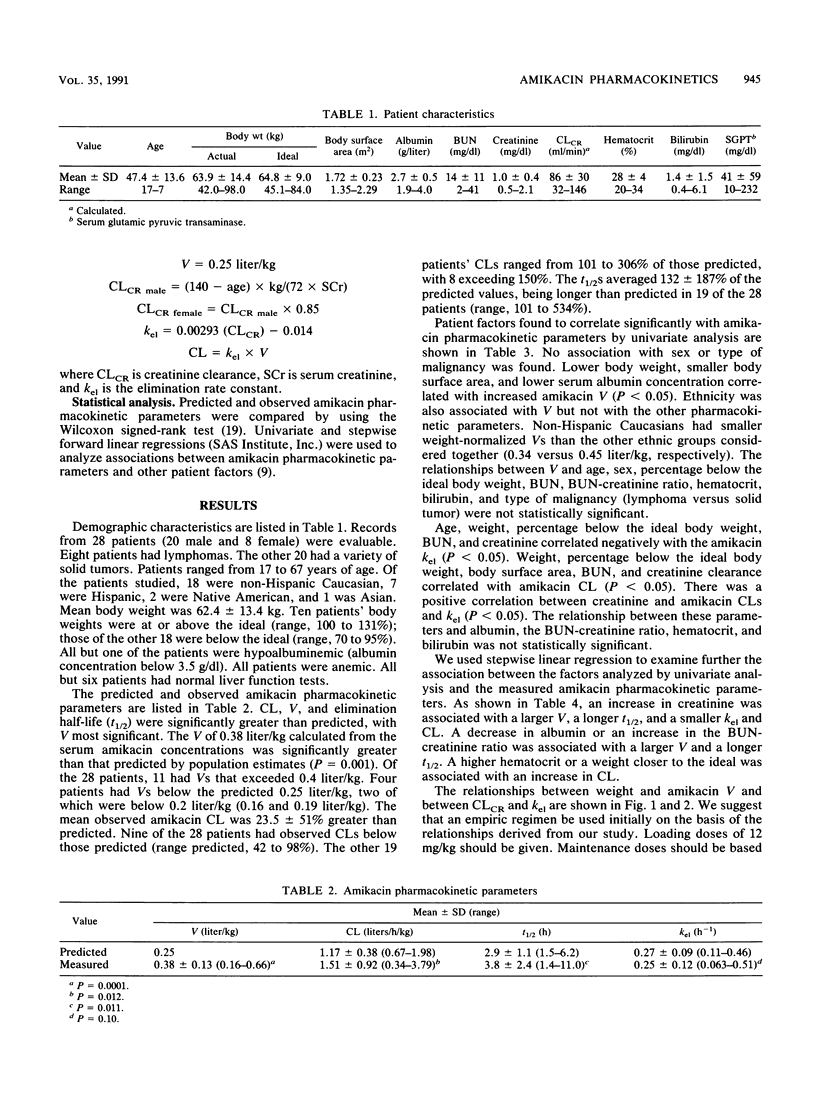
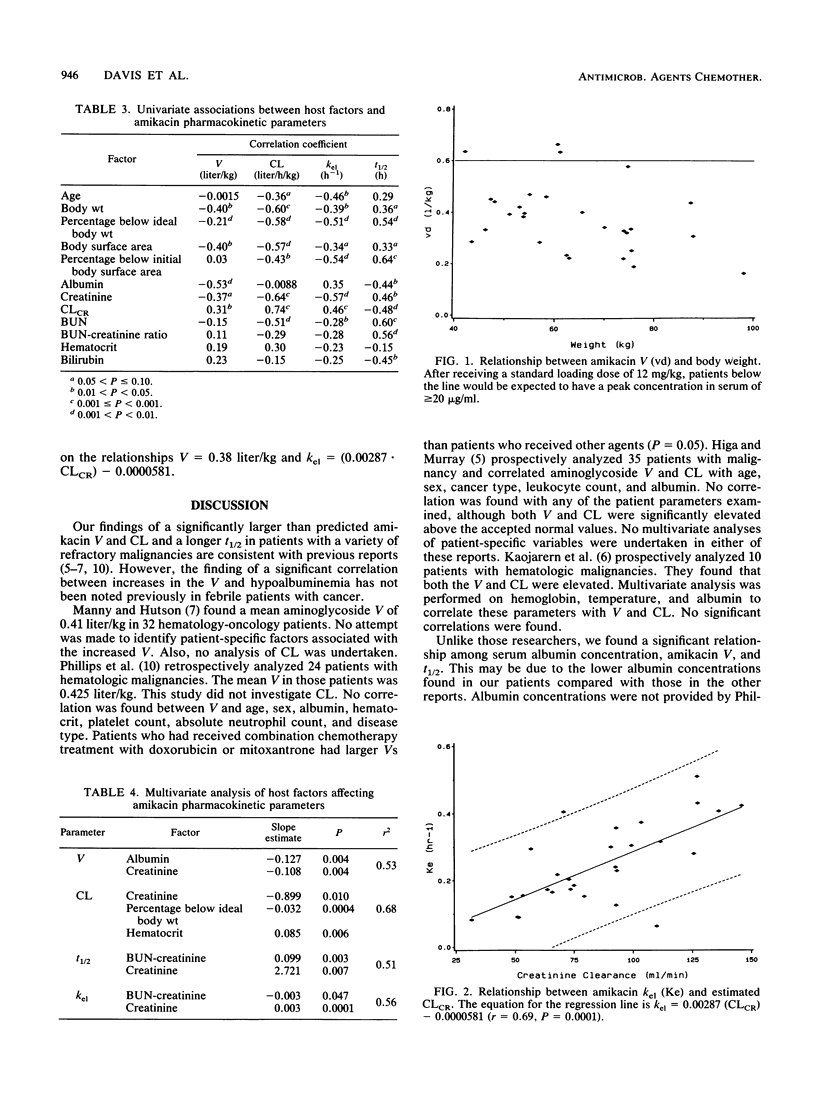
We retrospectively analyzed amikacin pharmacokinetics in 28 patients (mean age, 47.4 +/- 13.6 years) who received high-dose chemotherapy during a neutropenic febrile episode. Patients received an experimental protocol of high-dose anticancer chemotherapy. Amikacin pharmacokinetic parameters were calculated from two or more concentrations in serum around a single dose by the method of Sawchuck and Zaske (J. Pharmacokinet. Biopharm. 4:183-195, 1976). Predicted parameters were calculated by using standard methods. The observed amikacin volume of distribution and clearance were significantly greater and the elimination half-life was longer than predicted (0.38 +/- 0.13 versus 0.25 liter/kg [P = 0.0001], 1.51 +/- 0.92 versus 1.17 +/- 0.38 liters/h/kg [P = 0.012], and 3.8 +/- 2.4 versus 2.9 +/- 1.1 h [P = 0.011], respectively). Multivariate analysis revealed that albumin correlated negatively and creatinine correlated positively with the volume of distribution and the elimination half-life. Creatinine and the percentage below the ideal body weight correlated negatively and hematocrit correlated positively with clearance. Administration of dosage regimens based on predicted pharmacokinetic parameters yielded subtherapeutic amikacin concentrations in serum in our patients. Because of the increased dosage requirements and the need for adequate antibiotic treatment in this population, we suggest guidelines for empiric dosing for patients with advanced cancer receiving intensive chemotherapy.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Assael B. M., Parini R., Rusconi F. Ototoxicity of aminoglycoside antibiotics in infants and children. Pediatr Infect Dis. 1982 Sep-Oct;1(5):357–365. doi: 10.1097/00006454-198209000-00017. [DOI] [PubMed] [Google Scholar]
- Cockcroft D. W., Gault M. H. Prediction of creatinine clearance from serum creatinine. Nephron. 1976;16(1):31–41. doi: 10.1159/000180580. [DOI] [PubMed] [Google Scholar]
- Dickson C. J., Schwartzman M. S., Bertino J. S., Jr Factors affecting aminoglycoside disposition: effects of circadian rhythm and dietary protein intake on gentamicin pharmacokinetics. Clin Pharmacol Ther. 1986 Mar;39(3):325–328. doi: 10.1038/clpt.1986.47. [DOI] [PubMed] [Google Scholar]
- Higa G. M., Murray W. E. Alterations in aminoglycoside pharmacokinetics in patients with cancer. Clin Pharm. 1987 Dec;6(12):963–966. [PubMed] [Google Scholar]
- Kaojarern S., Maoleekoonpairoj S., Atichartakarn V. Pharmacokinetics of amikacin in hematologic malignancies. Antimicrob Agents Chemother. 1989 Aug;33(8):1406–1408. doi: 10.1128/aac.33.8.1406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manny R. P., Hutson P. R. Aminoglycoside volume of distribution in hematology-oncology patients. Clin Pharm. 1986 Aug;5(8):629–632. [PubMed] [Google Scholar]
- Moore R. D., Smith C. R., Lietman P. S. The association of aminoglycoside plasma levels with mortality in patients with gram-negative bacteremia. J Infect Dis. 1984 Mar;149(3):443–448. doi: 10.1093/infdis/149.3.443. [DOI] [PubMed] [Google Scholar]
- Phillips J. K., Spearing R. L., Crome D. J., Davies J. M. Gentamicin volumes of distribution in patients with hematologic disorders. N Engl J Med. 1988 Nov 10;319(19):1290–1290. doi: 10.1056/NEJM198811103191918. [DOI] [PubMed] [Google Scholar]
- Pizzo P. A., Commers J., Cotton D., Gress J., Hathorn J., Hiemenz J., Longo D., Marshall D., Robichaud K. J. Approaching the controversies in antibacterial management of cancer patients. Am J Med. 1984 Mar;76(3):436–449. doi: 10.1016/0002-9343(84)90663-6. [DOI] [PubMed] [Google Scholar]
- Powell S. H., Thompson W. L., Luthe M. A., Stern R. C., Grossniklaus D. A., Bloxham D. D., Groden D. L., Jacobs M. R., DiScenna A. O., Cash H. A. Once-daily vs. continuous aminoglycoside dosing: efficacy and toxicity in animal and clinical studies of gentamicin, netilmicin, and tobramycin. J Infect Dis. 1983 May;147(5):918–932. doi: 10.1093/infdis/147.5.918. [DOI] [PubMed] [Google Scholar]
- Riff L. J., Jackson G. G. Pharmacology of gentamicin in man. J Infect Dis. 1971 Dec;124 (Suppl):S98–105. doi: 10.1093/infdis/124.supplement_1.s98. [DOI] [PubMed] [Google Scholar]
- Sawchuk R. J., Zaske D. E., Cipolle R. J., Wargin W. A., Strate R. G. Kinetic model for gentamicin dosing with the use of individual patient parameters. Clin Pharmacol Ther. 1977 Mar;21(3):362–369. doi: 10.1002/cpt1977213362. [DOI] [PubMed] [Google Scholar]
- Sawchuk R. J., Zaske D. E. Pharmacokinetics of dosing regimens which utilize multiple intravenous infusions: gentamicin in burn patients. J Pharmacokinet Biopharm. 1976 Apr;4(2):183–195. doi: 10.1007/BF01086153. [DOI] [PubMed] [Google Scholar]
- Schentag J. J., Jusko W. J., Vance J. W., Cumbo T. J., Abrutyn E., DeLattre M., Gerbracht L. M. Gentamicin disposition and tissue accumulation on multiple dosing. J Pharmacokinet Biopharm. 1977 Dec;5(6):559–577. doi: 10.1007/BF01059684. [DOI] [PubMed] [Google Scholar]


