Abstract
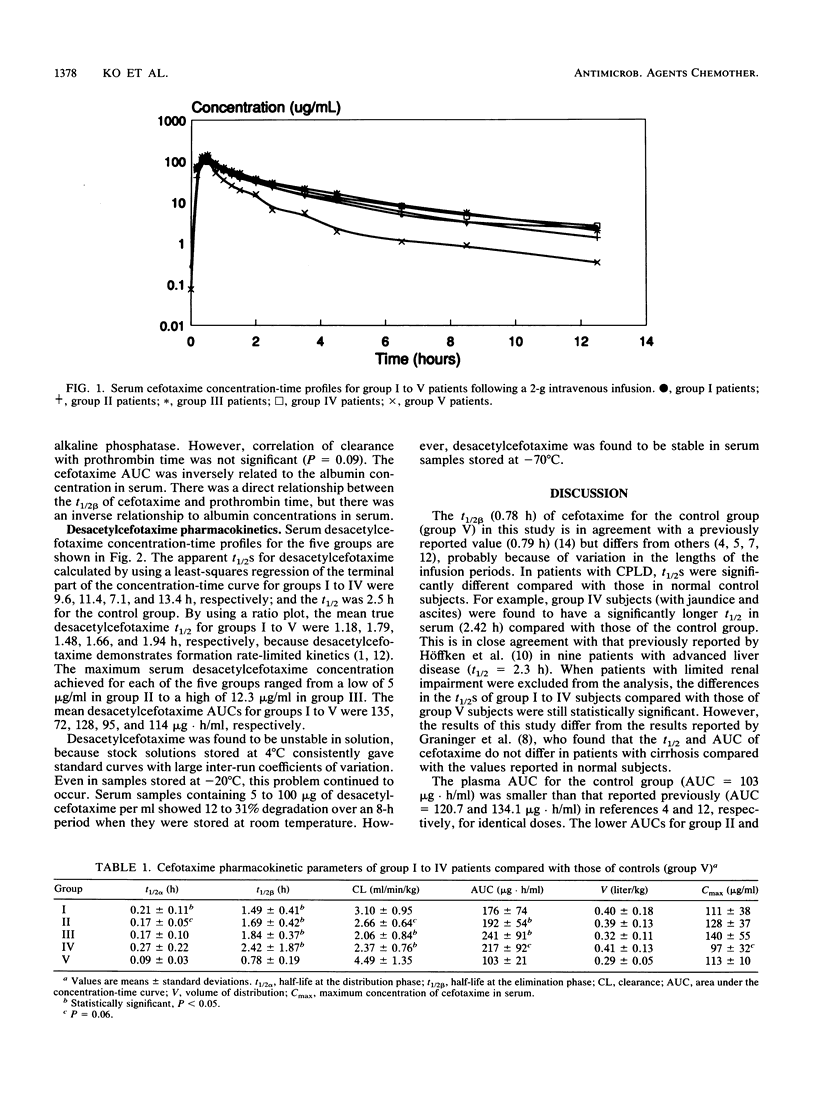
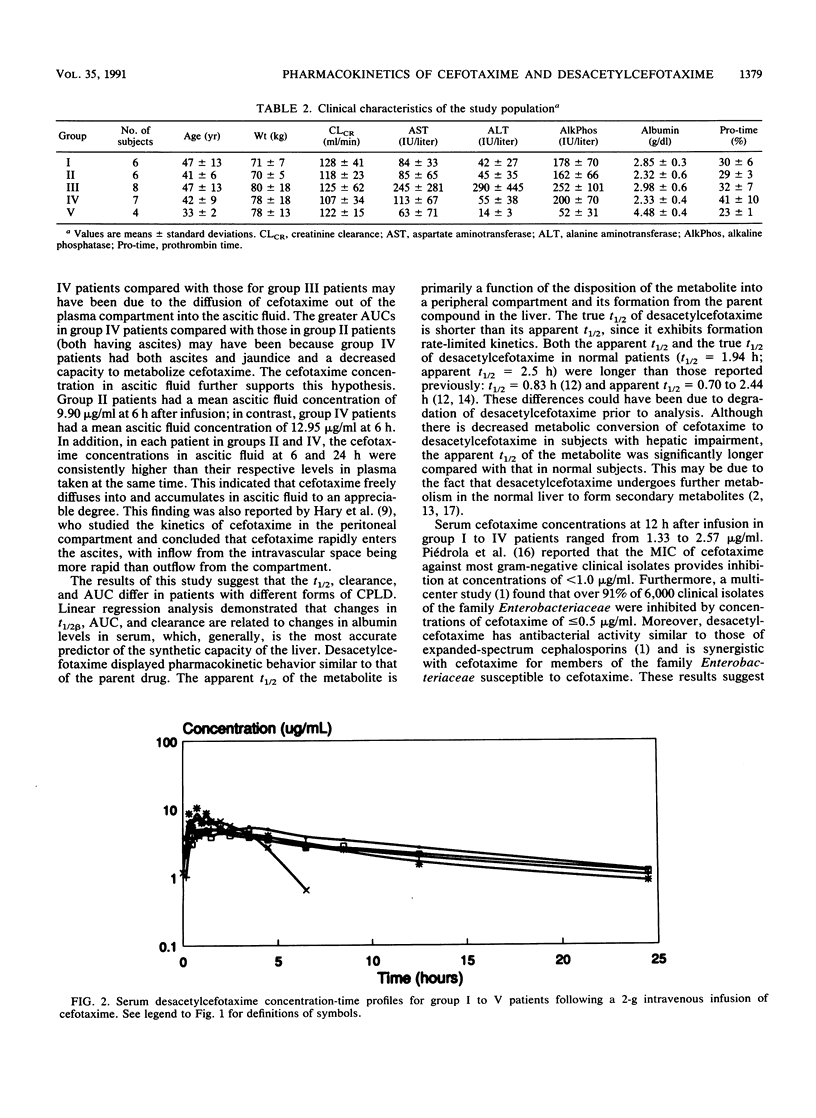
The dispositions of cefotaxime and its metabolite desacetylcefotaxime were investigated in patients with different forms of chronic parenchymal liver disease (CPLD). A total of 31 subjects (27 patients and 4 controls) received a single 2-g dose of cefotaxime by infusion, and serial blood samples were drawn. The area under the concentration-time curve ranged from 176 to 241 micrograms.h/ml, the apparent half-life ranged from 1.49 to 2.42 h, and clearance ranged from 2.06 to 3.10 ml/min/kg in patients with four different forms of CPLD. The area under the concentration-time curve and the apparent half-life of desacetylcefotaxime ranged from 72 to 128 micrograms.h/ml and 7.1 to 13.4 h, respectively. Pharmacokinetic parameters were significantly different in patients with CPLD compared with those in control subjects and were related to clinical indices of hepatic impairment. Modest accumulation of cefotaxime in patients with severe hepatic impairment is unlikely to produce toxicity because of its high therapeutic index, and dosing modifications may not be required.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Carmine A. A., Brogden R. N., Heel R. C., Speight T. M., Avery G. S. Cefotaxime. A review of its antibacterial activity, pharmacological properties and therapeutic use. Drugs. 1983 Mar;25(3):223–289. doi: 10.2165/00003495-198325030-00001. [DOI] [PubMed] [Google Scholar]
- Chamberlain J., Coombes J. D., Dell D., Fromson J. M., Ings R. J., Macdonald C. M., McEwen J. Metabolism of cefotaxime in animals and man. J Antimicrob Chemother. 1980 Sep;6 (Suppl A):69–78. doi: 10.1093/jac/6.suppl_a.69. [DOI] [PubMed] [Google Scholar]
- D'Argenio D. Z., Schumitzky A. A program package for simulation and parameter estimation in pharmacokinetic systems. Comput Programs Biomed. 1979 Mar;9(2):115–134. doi: 10.1016/0010-468x(79)90025-4. [DOI] [PubMed] [Google Scholar]
- Doluisio J. T. Clinical pharmacokinetics of cefotaxime in patients with normal and reduced renal function. Rev Infect Dis. 1982 Sep-Oct;4 (Suppl):S333–S345. doi: 10.1093/clinids/4.supplement_2.s333. [DOI] [PubMed] [Google Scholar]
- Esmieu F., Guibert J., Rosenkilde H. C., Ho I., Le Go A. Pharmacokinetics of cefotaxime in normal human volunteers. J Antimicrob Chemother. 1980 Sep;6 (Suppl A):83–92. doi: 10.1093/jac/6.suppl_a.83. [DOI] [PubMed] [Google Scholar]
- Fabre H., Kok W. T. Determination of cephalosporins and decomposition products by liquid chromatography with indirect electrochemical detection. Anal Chem. 1988 Jan 15;60(2):136–141. doi: 10.1021/ac00153a008. [DOI] [PubMed] [Google Scholar]
- Fillastre J. P., Leroy A., Humbert G., Godin M. Pharmacokinetics of cefotaxime in subjects with normal and impaired renal function. J Antimicrob Chemother. 1980 Sep;6 (Suppl A):103–111. doi: 10.1093/jac/6.suppl_a.103. [DOI] [PubMed] [Google Scholar]
- Graninger W., Uihlein M., Ferenci P., Moser C., Georgopoulos A. Cefotaxime and desacetyl-cefotaxime blood levels in hepatic dysfunction. J Antimicrob Chemother. 1984 Sep;14 (Suppl B):143–146. doi: 10.1093/jac/14.suppl_b.143. [DOI] [PubMed] [Google Scholar]
- Hary L., Andrejak M., Leleu S., Orfila J., Capron J. P. The pharmacokinetics of ceftriaxone and cefotaxime in cirrhotic patients with ascites. Eur J Clin Pharmacol. 1989;36(6):613–616. doi: 10.1007/BF00637745. [DOI] [PubMed] [Google Scholar]
- Houston J. B. Drug metabolite kinetics. Pharmacol Ther. 1981;15(3):521–552. doi: 10.1016/0163-7258(81)90056-5. [DOI] [PubMed] [Google Scholar]
- Höffken G., Lode H., Koeppe P., Ruhnke M., Borner K. Pharmacokinetics of cefotaxime and desacetyl-cefotaxime in cirrhosis of the liver. Chemotherapy. 1984;30(1):7–17. doi: 10.1159/000238238. [DOI] [PubMed] [Google Scholar]
- Ings R. M., Reeves D. S., White L. O., Bax R. P., Bywater M. J., Holt H. A. The human pharmacokinetics of cefotaxime and its metabolites and the role of renal tubular secretion on their elimination. J Pharmacokinet Biopharm. 1985 Apr;13(2):121–142. doi: 10.1007/BF01059394. [DOI] [PubMed] [Google Scholar]
- Lassman H. B., Coombes J. D. Metabolism of cefotaxime: a review. Diagn Microbiol Infect Dis. 1984 Jun;2(3 Suppl):3S–12S. [PubMed] [Google Scholar]
- Matzke G. R., Abraham P. A., Halstenson C. E., Keane W. F. Cefotaxime and desacetyl cefotaxime kinetics in renal impairment. Clin Pharmacol Ther. 1985 Jul;38(1):31–36. doi: 10.1038/clpt.1985.130. [DOI] [PubMed] [Google Scholar]
- Paré P., Talbot J., Hoefs J. C. Serum-ascites albumin concentration gradient: a physiologic approach to the differential diagnosis of ascites. Gastroenterology. 1983 Aug;85(2):240–244. [PubMed] [Google Scholar]
- Piédrola G., Galan I., Leyva A., Maroto M. C. Comparison of in vitro activity of cefotaxime and desacetylcefotaxime alone and in combination against 320 gram-negative clinical isolates. Drugs. 1988;35 (Suppl 2):62–64. doi: 10.2165/00003495-198800352-00014. [DOI] [PubMed] [Google Scholar]
- Reeves D. S., White L. O., Holt H. A., Bahari D., Bywater M. J., Bax R. P. Human metabolism of cefotaxime. J Antimicrob Chemother. 1980 Sep;6 (Suppl A):93–101. doi: 10.1093/jac/6.suppl_a.93. [DOI] [PubMed] [Google Scholar]
- Runyon B. A. Spontaneous bacterial peritonitis: an explosion of information. Hepatology. 1988 Jan-Feb;8(1):171–175. doi: 10.1002/hep.1840080131. [DOI] [PubMed] [Google Scholar]
- Wise R., Wright N. The pharmacokinetics of cefotaxime and ceftriaxone in renal and hepatic dysfunction. Infection. 1985;13 (Suppl 1):S145–S150. doi: 10.1007/BF01644237. [DOI] [PubMed] [Google Scholar]
- Wise R., Wright N., Wills P. J. Pharmacology of cefotaxime and its desacetyl metabolite in renal and hepatic disease. Antimicrob Agents Chemother. 1981 Apr;19(4):526–531. doi: 10.1128/aac.19.4.526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamaoka K., Nakagawa T., Uno T. Application of Akaike's information criterion (AIC) in the evaluation of linear pharmacokinetic equations. J Pharmacokinet Biopharm. 1978 Apr;6(2):165–175. doi: 10.1007/BF01117450. [DOI] [PubMed] [Google Scholar]


