Abstract
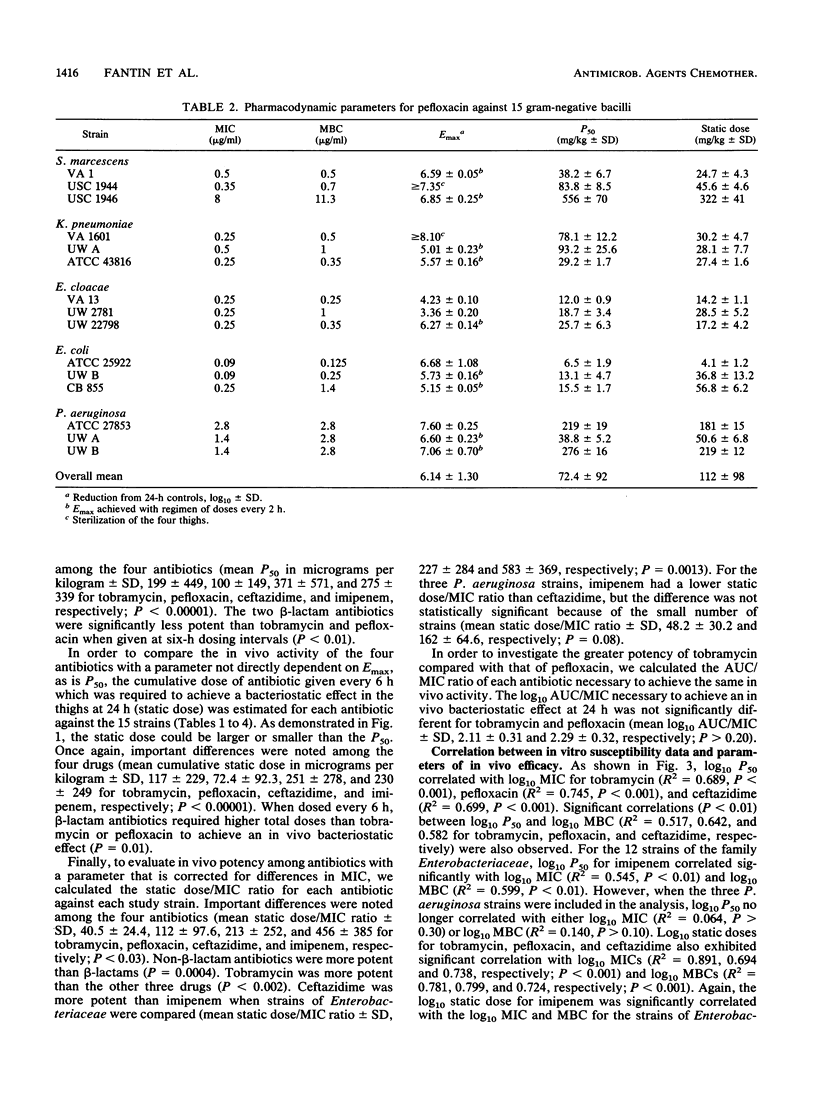
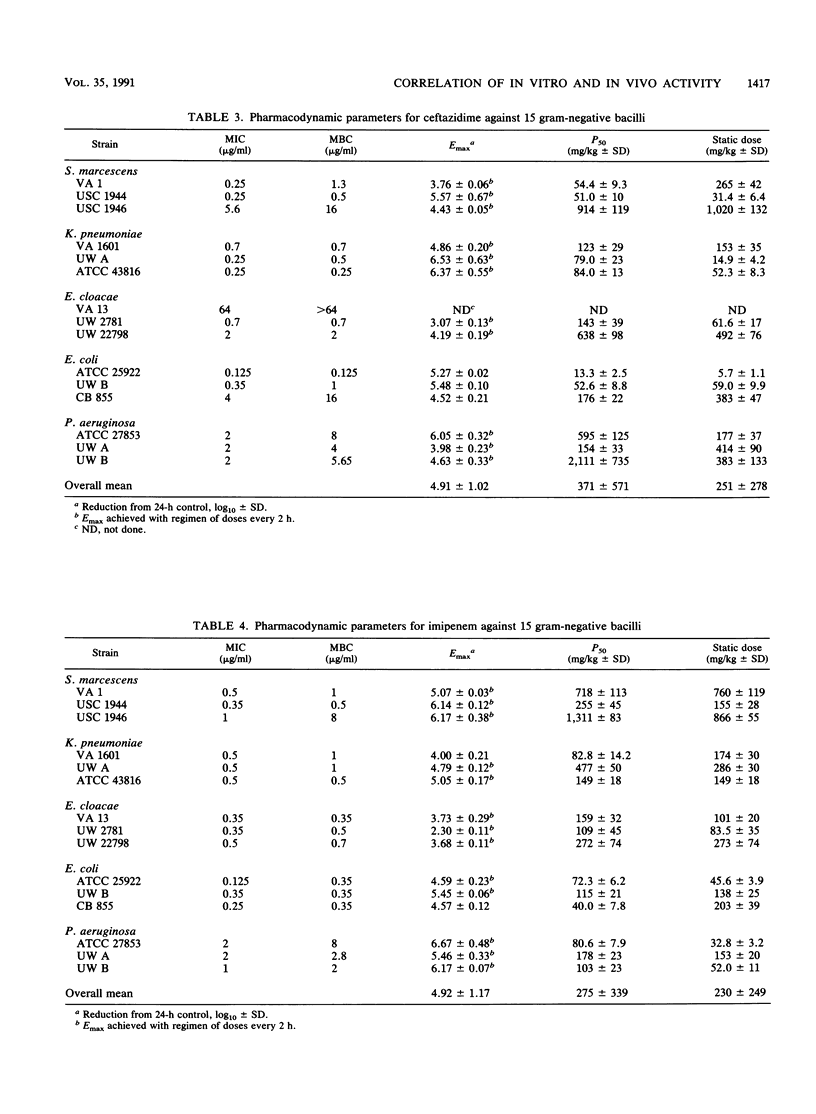
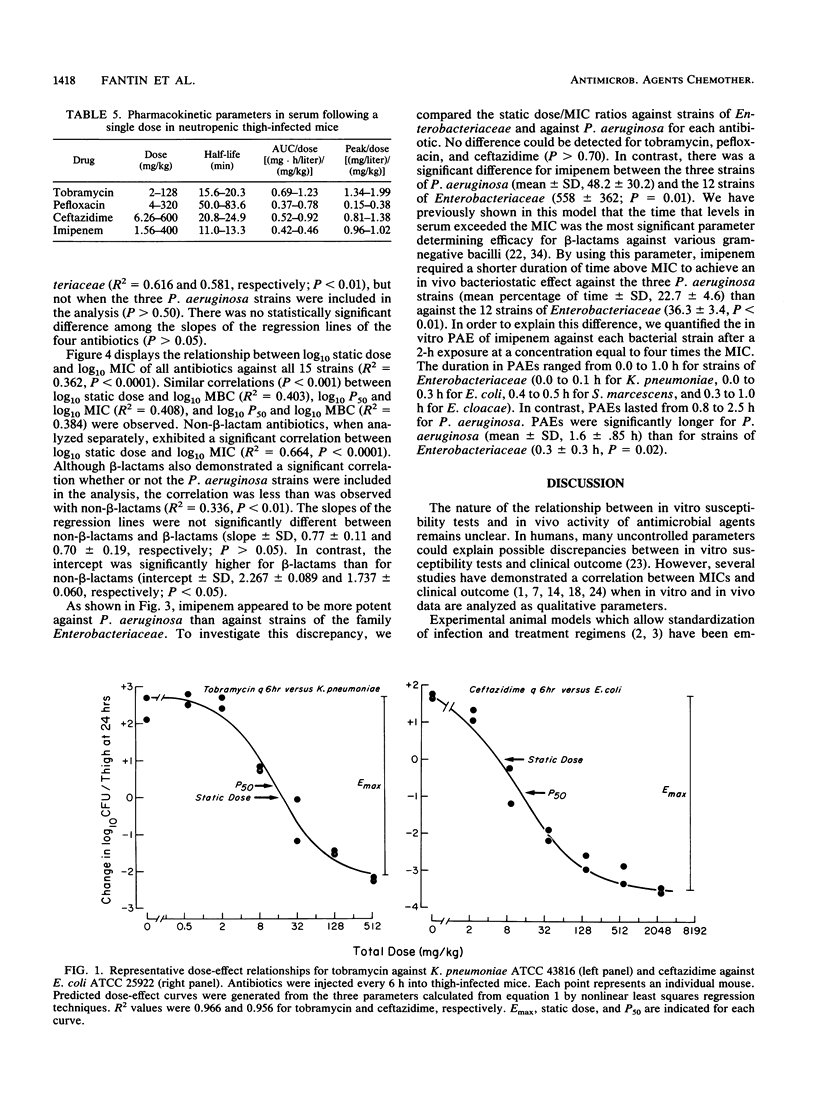
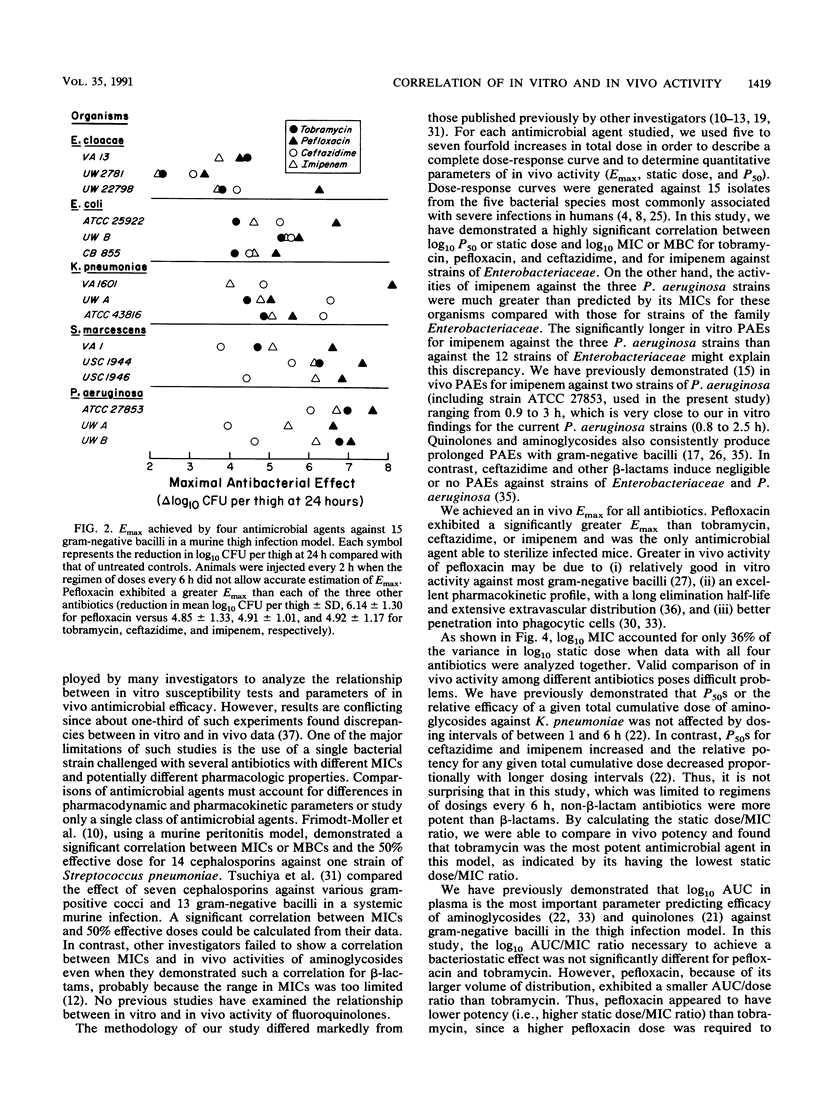
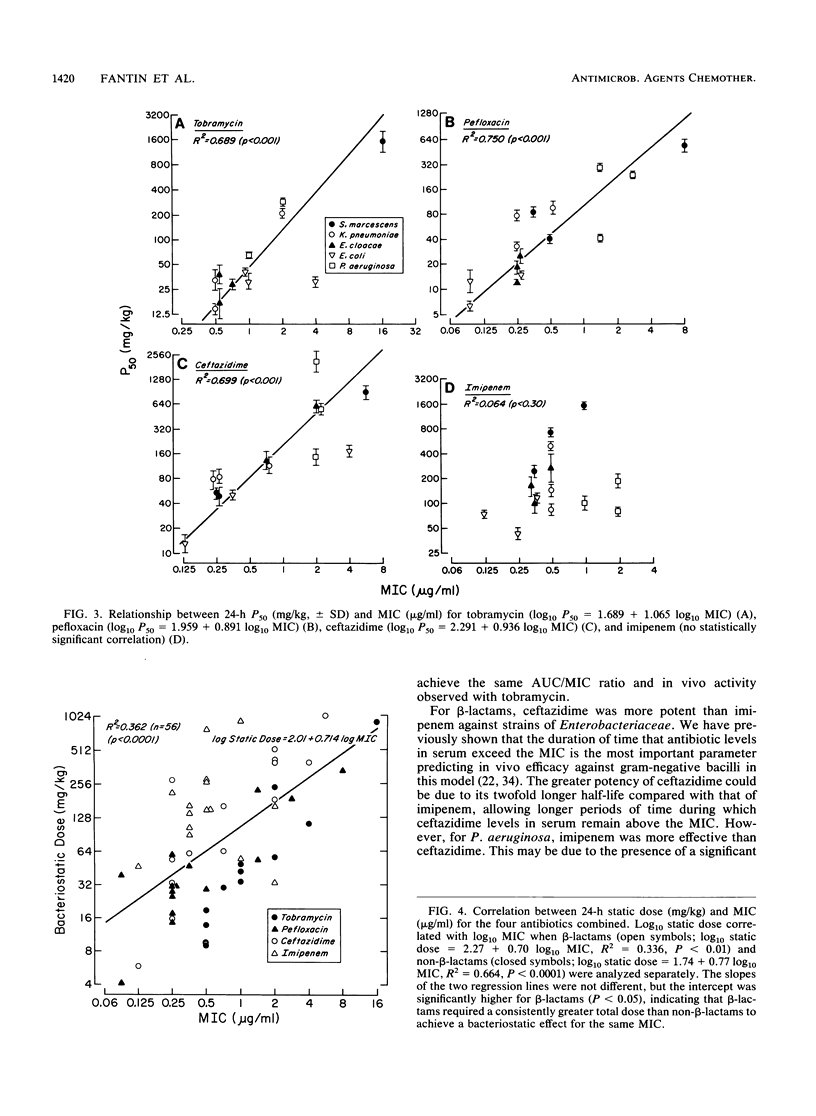
We studied the relationship between in vitro susceptibility tests (MICs, MBCs) and in vivo activity of tobramycin, pefloxacin, ceftazidime, and imipenem against 15 gram-negative bacilli from five different species in a murine thigh infection model. Complete dose-response curves were determined for each antimicrobial agent against each strain, and three parameters of in vivo activity were defined: maximal attainable antimicrobial effect (i.e., reduction in log10 CFU per thigh compared with untreated controls) at 24 h (Emax), total dose required to reach 50% of maximal effect (P50), and total dose required to achieve a bacteriostatic effect (static dose). Pefloxacin demonstrated the greatest Emax (P less than 0.05). Tobramycin was the most potent antimicrobial agent, as indicated by its having the lowest static dose/MIC ratio (P less than 0.002). Log10 P50s and static doses correlated significantly with log10 MICs or MBCs for the 15 strains of each antibiotic (P less than 0.01) except imipenem (P greater than 0.50). The greater potency of imipenem against the three Pseudomonas aeruginosa strains than against strains of the family Enterobacteriaceae (P less than 0.01) explained this lack of correlation. A longer duration of postantibiotic effect for imipenem against P. aeruginosa (P = 0.02) contributed to its increased potency against these strains. We conclude that in vitro susceptibility tests correlated well with in vivo activity in this animal model and that variations in potency among the four antimicrobial agents could be explained by differences in pharmacokinetics or pharmacodynamic activity.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- ABBOUD F. M., WAISBREN B. A. Correlation between in vitro studies and response to antibiotic therapy in staphylococcic bacteremia. AMA Arch Intern Med. 1959 Aug;104(2):226–233. doi: 10.1001/archinte.1959.00270080052006. [DOI] [PubMed] [Google Scholar]
- Barza M. A critique of animal models in antibiotic research. Scand J Infect Dis Suppl. 1978;(14):109–117. [PubMed] [Google Scholar]
- Bergeron M. G. A review of models for the therapy of experimental infections. Scand J Infect Dis Suppl. 1978;(14):189–206. [PubMed] [Google Scholar]
- Bryant R. E., Hood A. F., Hood C. E., Koenig M. G. Factors affecting mortality of gram-negative rod bacteremia. Arch Intern Med. 1971 Jan;127(1):120–128. [PubMed] [Google Scholar]
- Bustamante C. I., Drusano G. L., Tatem B. A., Standiford H. C. Postantibiotic effect of imipenem on Pseudomonas aeruginosa. Antimicrob Agents Chemother. 1984 Nov;26(5):678–682. doi: 10.1128/aac.26.5.678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CURTIS F. R., WILKINSON A. E. A comparison of the in vitro sensitivity of gonococci to penicillin with the results of treatment. Br J Vener Dis. 1958 Jun;34(2):70–82. doi: 10.1136/sti.34.2.70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DuPont H. L., Spink W. W. Infections due to gram-negative organisms: an analysis of 860 patients with bacteremia at the University of Minnesota Medical Center, 1958-1966. Medicine (Baltimore) 1969 Jul;48(4):307–332. doi: 10.1097/00005792-196907000-00003. [DOI] [PubMed] [Google Scholar]
- Frimodt-Møller N., Bentzon M. W., Thomsen V. F. Experimental infection with Streptococcus pneumoniae in mice: correlation of in vitro activity and pharmacokinetic parameters with in vivo effect for 14 cephalosporins. J Infect Dis. 1986 Sep;154(3):511–517. doi: 10.1093/infdis/154.3.511. [DOI] [PubMed] [Google Scholar]
- Frimodt-Møller N., Bentzon M. W., Thomsen V. F. Experimental pneumococcus infection in mice: comparative in vitro and in vivo effect of cefuroxime, cefotaxime and ceftriaxone. Acta Pathol Microbiol Immunol Scand B. 1987 Oct;95(5):261–267. doi: 10.1111/j.1699-0463.1987.tb03123.x. [DOI] [PubMed] [Google Scholar]
- Frimodt-Møller N., Thomsen V. F. Experimental pneumococcus infection in mice: correlation of bactericidal activity in vitro with the effect in vivo for gentamicin, netilmicin and tobramycin. Acta Pathol Microbiol Immunol Scand B. 1987 Jun;95(3):153–158. doi: 10.1111/j.1699-0463.1987.tb03105.x. [DOI] [PubMed] [Google Scholar]
- Frimodt-Møller N., Thomsen V. F. The pneumococcus and the mouse-protection test: correlation of in vitro and in vivo activity for beta-lactam antibiotics, vancomycin, erythromycin and gentamicin. Acta Pathol Microbiol Immunol Scand B. 1987 Jun;95(3):159–165. doi: 10.1111/j.1699-0463.1987.tb03106.x. [DOI] [PubMed] [Google Scholar]
- Gerber A. U., Craig W. A. Worldwide clinical experience with cefoperazone. Drugs. 1981;22 (Suppl 1):108–118. doi: 10.2165/00003495-198100221-00022. [DOI] [PubMed] [Google Scholar]
- Gudmundsson S., Vogelman B., Craig W. A. The in-vivo postantibiotic effect of imipenem and other new antimicrobials. J Antimicrob Chemother. 1986 Dec;18 (Suppl E):67–73. doi: 10.1093/jac/18.supplement_e.67. [DOI] [PubMed] [Google Scholar]
- Holford N. H., Sheiner L. B. Understanding the dose-effect relationship: clinical application of pharmacokinetic-pharmacodynamic models. Clin Pharmacokinet. 1981 Nov-Dec;6(6):429–453. doi: 10.2165/00003088-198106060-00002. [DOI] [PubMed] [Google Scholar]
- Ingerman M. J., Pitsakis P. G., Rosenberg A. F., Levison M. E. The importance of pharmacodynamics in determining the dosing interval in therapy for experimental pseudomonas endocarditis in the rat. J Infect Dis. 1986 Apr;153(4):707–714. doi: 10.1093/infdis/153.4.707. [DOI] [PubMed] [Google Scholar]
- Joiner K., Lowe B., Dzink J., Bartlett J. G. Comparative efficacy of 10 antimicrobial agents in experimental infections with Bacteroides fragilis. J Infect Dis. 1982 Apr;145(4):561–568. doi: 10.1093/infdis/145.4.561. [DOI] [PubMed] [Google Scholar]
- Kreger B. E., Craven D. E., McCabe W. R. Gram-negative bacteremia. IV. Re-evaluation of clinical features and treatment in 612 patients. Am J Med. 1980 Mar;68(3):344–355. doi: 10.1016/0002-9343(80)90102-3. [DOI] [PubMed] [Google Scholar]
- Leggett J. E., Ebert S., Fantin B., Craig W. A. Comparative dose-effect relations at several dosing intervals for beta-lactam, aminoglycoside and quinolone antibiotics against gram-negative bacilli in murine thigh-infection and pneumonitis models. Scand J Infect Dis Suppl. 1990;74:179–184. [PubMed] [Google Scholar]
- Leggett J. E., Fantin B., Ebert S., Totsuka K., Vogelman B., Calame W., Mattie H., Craig W. A. Comparative antibiotic dose-effect relations at several dosing intervals in murine pneumonitis and thigh-infection models. J Infect Dis. 1989 Feb;159(2):281–292. doi: 10.1093/infdis/159.2.281. [DOI] [PubMed] [Google Scholar]
- Moore R. D., Lietman P. S., Smith C. R. Clinical response to aminoglycoside therapy: importance of the ratio of peak concentration to minimal inhibitory concentration. J Infect Dis. 1987 Jan;155(1):93–99. doi: 10.1093/infdis/155.1.93. [DOI] [PubMed] [Google Scholar]
- Myerowitz R. L., Medeiros A. A., O'Brien T. F. Recent experience with bacillemia due to gram-negative organisms. J Infect Dis. 1971 Sep;124(3):239–246. doi: 10.1093/infdis/124.3.239. [DOI] [PubMed] [Google Scholar]
- Neu H. C., Kumada T., Chin N. X., Mandell W. The post-antimicrobial suppressive effect of quinolone agents. Drugs Exp Clin Res. 1987;13(2):63–67. [PubMed] [Google Scholar]
- Phillips I., King A. Comparative activity of the 4-quinolones. Rev Infect Dis. 1988 Jan-Feb;10 (Suppl 1):S70–S76. doi: 10.1093/clinids/10.supplement_1.s70. [DOI] [PubMed] [Google Scholar]
- RODGER K. C., BRANCH A., POWER E. E., STARKEY D. H., GREGORY E., MURRAY R. D., HARROP J. Antibiotic therapy: correlation of clinical results with laboratory sensitivity tests. Can Med Assoc J. 1956 Apr 15;74(8):605–612. [PMC free article] [PubMed] [Google Scholar]
- Traub W. H. Intraphagocytic bactericidal activity of bacterial DNA gyrase inhibitors against Serratia marcescens. Chemotherapy. 1984;30(6):379–386. doi: 10.1159/000238297. [DOI] [PubMed] [Google Scholar]
- Tsuchiya K., Kondo M., Kida M., Nakao M., Iwahi T., Nishi T., Noji Y., Takeuchi M., Nozaki Y. Cefmenoxime (SCE-1365), a novel broad-spectrum cephalosporin: in vitro and in vivo antibacterial activities. Antimicrob Agents Chemother. 1981 Jan;19(1):56–65. doi: 10.1128/aac.19.1.56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Unadkat J. D., Bartha F., Sheiner L. B. Simultaneous modeling of pharmacokinetics and pharmacodynamics with nonparametric kinetic and dynamic models. Clin Pharmacol Ther. 1986 Jul;40(1):86–93. doi: 10.1038/clpt.1986.143. [DOI] [PubMed] [Google Scholar]
- Van der Auwera P., Matsumoto T., Husson M. Intraphagocytic penetration of antibiotics. J Antimicrob Chemother. 1988 Aug;22(2):185–192. doi: 10.1093/jac/22.2.185. [DOI] [PubMed] [Google Scholar]
- Vogelman B., Gudmundsson S., Turnidge J., Leggett J., Craig W. A. In vivo postantibiotic effect in a thigh infection in neutropenic mice. J Infect Dis. 1988 Feb;157(2):287–298. doi: 10.1093/infdis/157.2.287. [DOI] [PubMed] [Google Scholar]
- Wise R., Lister D., McNulty C. A., Griggs D., Andrews J. M. The comparative pharmacokinetics of five quinolones. J Antimicrob Chemother. 1986 Nov;18 (Suppl 500):71–81. doi: 10.1093/jac/18.supplement_d.71. [DOI] [PubMed] [Google Scholar]


