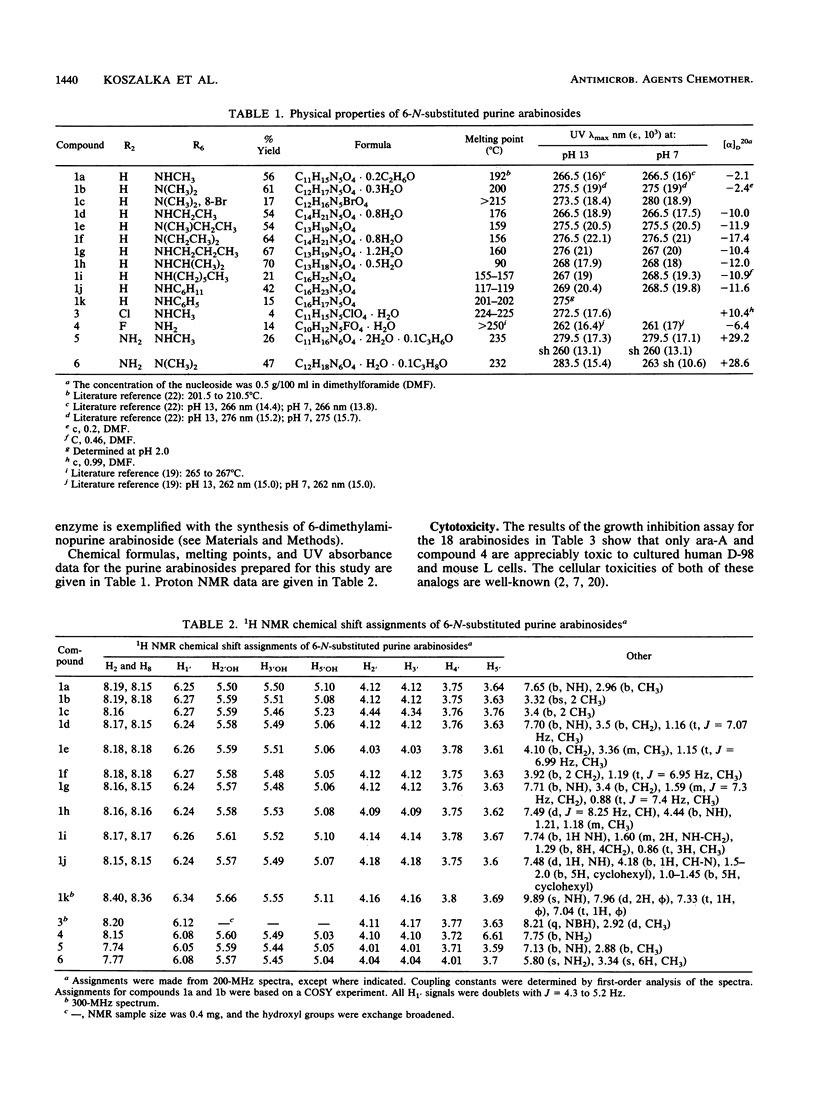
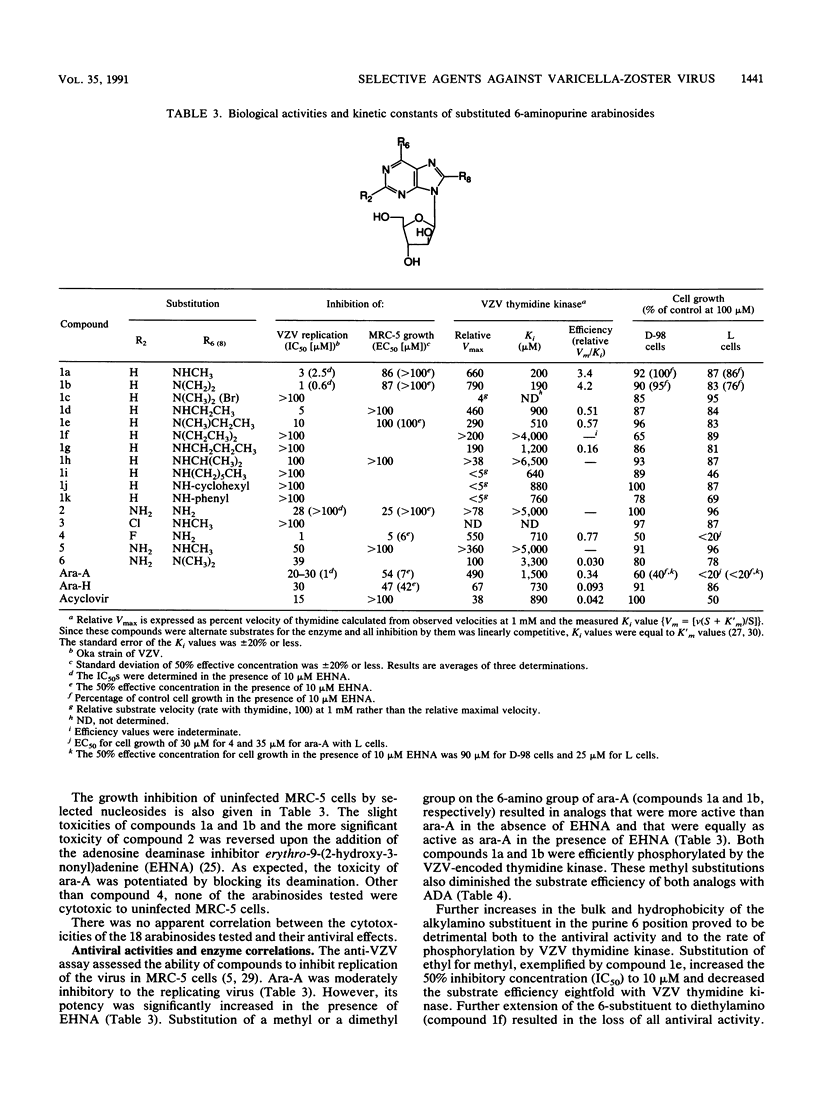
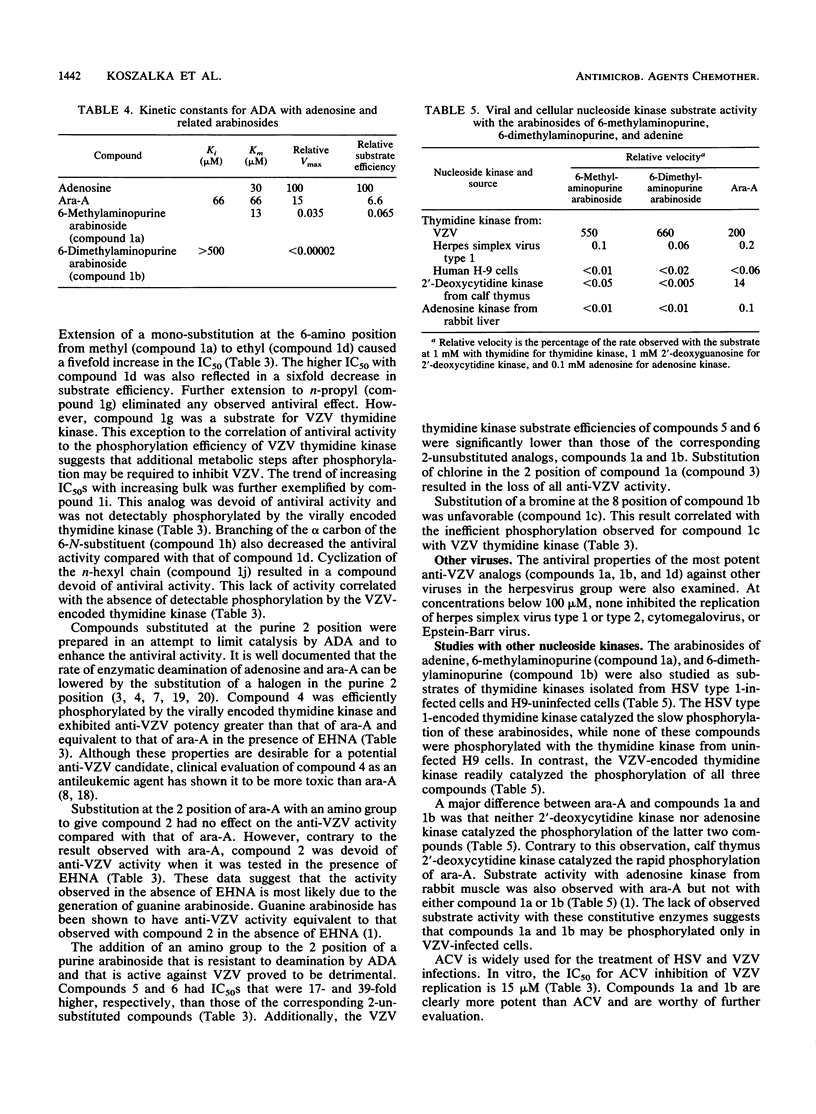
Abstract
A series of 6-alkylaminopurine arabinosides were synthesized and found to inhibit varicella-zoster virus (VZV). The antiviral activities of these nucleosides were limited to VZV. None of the other viruses tested in the herpesvirus family were affected. The in vitro antiviral potencies of the 18 arabinosides correlated with their efficiencies as substrates of the VZV-encoded thymidine kinase in all but one case. The arabinosides of 6-methylaminopurine and 6-dimethylaminopurine were the most potent analogs, with 50% inhibitory concentrations against VZV of 3 and 1 microM, respectively. They were not cytotoxic to uninfected MRC-5 cells, human Detroit 98 cells, or mouse L cells (50% inhibitory concentration, greater than 100 microM). Neither 6-methylaminopurine arabinoside nor 6-dimethylaminopurine arabinoside was detectably phosphorylated by either adenosine kinase or 2'-deoxycytidine kinase. These two alkylaminopurine arabinosides were also resistant to deamination catalyzed by adenosine deaminase. The VZV-dependent phosphorylation of these nucleosides offers the possibility of a potent and highly selective therapy for VZV infection.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Averett D. R., Koszalka G. W., Fyfe J. A., Roberts G. B., Purifoy D. J., Krenitsky T. A. 6-Methoxypurine arabinoside as a selective and potent inhibitor of varicella-zoster virus. Antimicrob Agents Chemother. 1991 May;35(5):851–857. doi: 10.1128/aac.35.5.851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Avramis V. I., Plunkett W. Metabolism and therapeutic efficacy of 9-beta-D-arabinofuranosyl-2-fluoroadenine against murine leukemia P388. Cancer Res. 1982 Jul;42(7):2587–2591. [PubMed] [Google Scholar]
- Baer H. P., Drummond G. I., Duncan E. L. Formation and deamination of adenosine by cardiac muscle enzymes. Mol Pharmacol. 1966 Jan;2(1):67–76. [PubMed] [Google Scholar]
- Berkowitz F. E., Levin M. J. Use of an enzyme-linked immunosorbent assay performed directly on fixed infected cell monolayers for evaluating drugs against varicella-zoster virus. Antimicrob Agents Chemother. 1985 Aug;28(2):207–210. doi: 10.1128/aac.28.2.207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Biron K. K., Elion G. B. In vitro susceptibility of varicella-zoster virus to acyclovir. Antimicrob Agents Chemother. 1980 Sep;18(3):443–447. doi: 10.1128/aac.18.3.443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brockman R. W., Schabel F. M., Jr, Montgomery J. A. Biologic activity of 9-beta-D-arabinofuranosyl-2-fluoroadenine, a metabolically stable analog of 9-beta-D-arabinofuranosyladenine. Biochem Pharmacol. 1977 Nov 15;26(22):2193–2196. doi: 10.1016/0006-2952(77)90275-1. [DOI] [PubMed] [Google Scholar]
- Chun H. G., Leyland-Jones B. R., Caryk S. M., Hoth D. F. Central nervous system toxicity of fludarabine phosphate. Cancer Treat Rep. 1986 Oct;70(10):1225–1228. [PubMed] [Google Scholar]
- Fyfe J. A., Keller P. M., Furman P. A., Miller R. L., Elion G. B. Thymidine kinase from herpes simplex virus phosphorylates the new antiviral compound, 9-(2-hydroxyethoxymethyl)guanine. J Biol Chem. 1978 Dec 25;253(24):8721–8727. [PubMed] [Google Scholar]
- Machida H. Comparison of susceptibilities of varicella-zoster virus and herpes simplex viruses to nucleoside analogs. Antimicrob Agents Chemother. 1986 Mar;29(3):524–526. doi: 10.1128/aac.29.3.524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Merkel D. E., Griffin N. L., Kagan-Hallet K., Von Hoff D. D. Central nervous system toxicity with fludarabine. Cancer Treat Rep. 1986 Dec;70(12):1449–1450. [PubMed] [Google Scholar]
- Montgomery J. A., Hewson K. Nucleosides of 2-fluoroadenine. J Med Chem. 1969 May;12(3):498–504. doi: 10.1021/jm00303a605. [DOI] [PubMed] [Google Scholar]
- Plunkett W., Chubb S., Alexander L., Montgomery J. A. Comparison of the toxicity and metabolism of 9-beta-D-arabinofuranosyl-2-fluoroadenine and 9-beta-D-arabinofuranosyladenine in human lymphoblastoid cells. Cancer Res. 1980 Jul;40(7):2349–2355. [PubMed] [Google Scholar]
- Prober C. G., Kirk L. E., Keeney R. E. Acyclovir therapy of chickenpox in immunosuppressed children--a collaborative study. J Pediatr. 1982 Oct;101(4):622–625. doi: 10.1016/s0022-3476(82)80725-7. [DOI] [PubMed] [Google Scholar]
- Rideout J. L., Krenitsky T. A., Koszalka G. W., Cohn N. K., Chao E. Y., Elion G. B., Latter V. S., Williams R. B. Pyrazolo[3,4-d]pyrimidine ribonucleosides as anticoccidials. 2. Synthesis and activity of some nucleosides of 4-(alkylamino)-1H-pyrazolo[3, 4-d]pyrimidines. J Med Chem. 1982 Sep;25(9):1040–1044. doi: 10.1021/jm00351a007. [DOI] [PubMed] [Google Scholar]
- Sacks S. L., Smith J. L., Pollard R. B., Sawhney V., Mahol A. S., Gregory P., Merigan T. C., Robinson W. S. Toxicity of vidarabine. JAMA. 1979 Jan 5;241(1):28–29. doi: 10.1001/jama.1979.03290270020010. [DOI] [PubMed] [Google Scholar]
- Schaeffer H. J., Schwender C. F. Enzyme inhibitors. 26. Bridging hydrophobic and hydrophilic regions on adenosine deaminase with some 9-(2-hydroxy-3-alkyl)adenines. J Med Chem. 1974 Jan;17(1):6–8. doi: 10.1021/jm00247a002. [DOI] [PubMed] [Google Scholar]
- Secrist J. A., 3rd, Shortnacy A. T., Montgomery J. A. Synthesis and biological evaluations of certain 2-halo-2'-substituted derivatives of 9-beta-D-arabinofuranosyladenine. J Med Chem. 1988 Feb;31(2):405–410. doi: 10.1021/jm00397a024. [DOI] [PubMed] [Google Scholar]
- Simon L. N., Bauer R. J., Tolman R. L., Robins R. K. Calf intestine adenosine deaminase. Substrate specificity. Biochemistry. 1970 Feb 3;9(3):573–577. doi: 10.1021/bi00805a018. [DOI] [PubMed] [Google Scholar]
- Spector T. Progress curve analysis of adenosine deaminase-catalyzed reactions. Anal Biochem. 1984 Apr;138(1):242–245. doi: 10.1016/0003-2697(84)90796-6. [DOI] [PubMed] [Google Scholar]
- Spector T., Stonehuerner J. G., Biron K. K., Averett D. R. Ribonucleotide reductase induced by varicella zoster virus. Characterization, and potentiation of acyclovir by its inhibition. Biochem Pharmacol. 1987 Dec 15;36(24):4341–4346. doi: 10.1016/0006-2952(87)90682-4. [DOI] [PubMed] [Google Scholar]
- Whitley R. J., Ch'ien L. T., Dolin R., Galasso G. J., Alford C. A., Jr Adenine arabinoside therapy of herpes zoster in the immunosuppressed. NIAID collaborative antiviral study. N Engl J Med. 1976 May 27;294(22):1193–1199. doi: 10.1056/NEJM197605272942201. [DOI] [PubMed] [Google Scholar]


