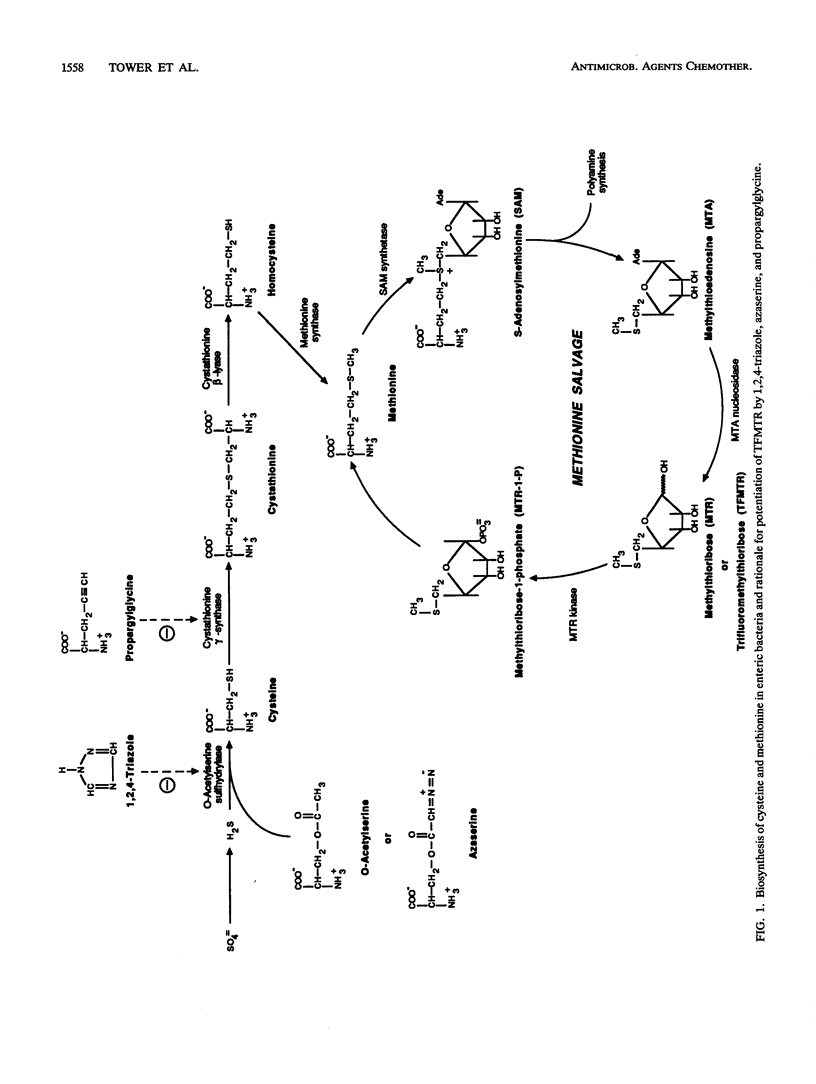
Abstract
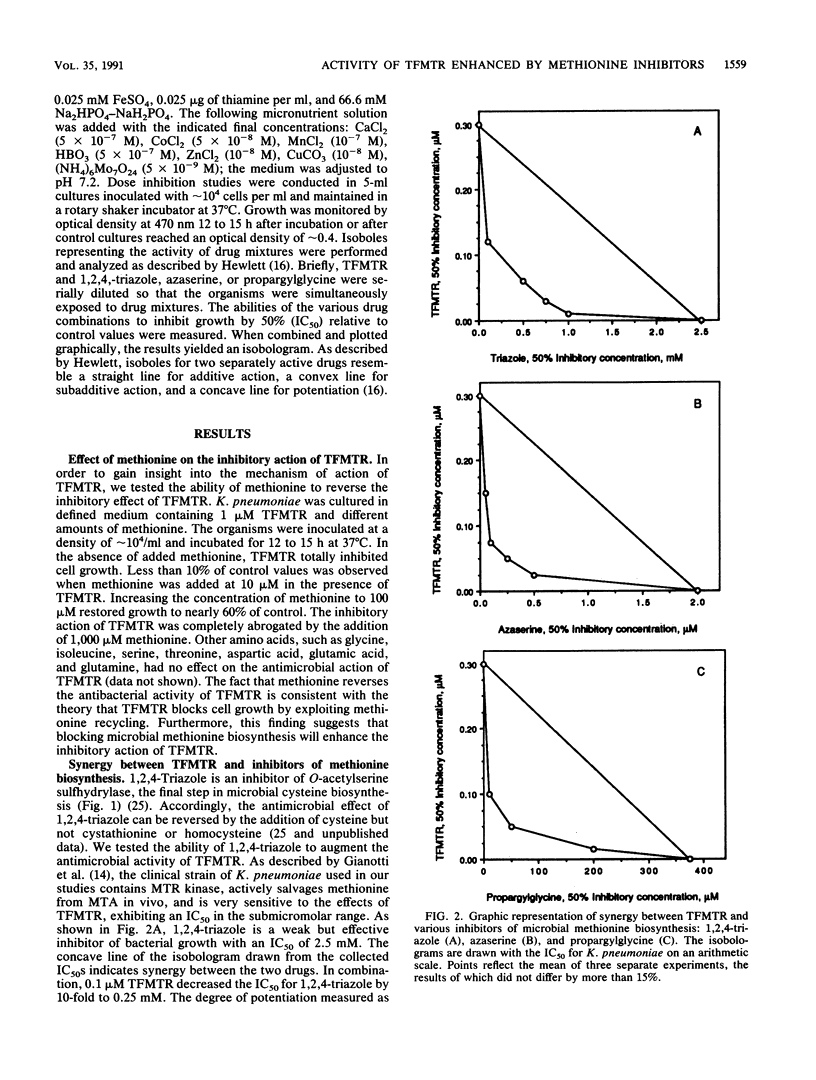
5-Methylthioribose (MTR) is an intermediate in the methionine recycling pathway of organisms containing the enzyme MTR kinase. Analogs of MTR have been proposed as a new class of antimicrobial agents because of their ability to perturb the growth of MTR kinase-containing pathogens through inhibition of methionine salvage or by conversion to toxic products. One such analog, 5-trifluoromethylthioribose (TFMTR), has demonstrated potent inhibitory effects on the growth of Klebsiella pneumoniae (A. G. Gianotti, P. A. Tower, J. H. Sheley, P. A. Conte, C. Spiro, J. H. Fitchen, and M. K. Riscoe, J. Biol. Chem. 265:831-837, 1990). Although the mode of action of TFMTR has yet to be determined, it is believed that the drug is converted to the toxic products trifluoromethionine or carbonothioic difluoride via MTR kinase and the methionine recycling pathway. On the basis of this assumption, we theorized that blocking de novo methionine synthesis would increase dependence on the methionine salvage pathway and lead to an increased rate of synthesis of toxic metabolites from TFMTR. In this report, we show that three separate inhibitors of de novo methionine synthesis (1,2,4-triazole, azaserine, and propargylglycine) act synergistically with TFMTR in inhibiting the growth of K. pneumoniae.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Backlund P. S., Jr, Chang C. P., Smith R. A. Identification of 2-keto-4-methylthiobutyrate as an intermediate compound in methionine synthesis from 5'-methylthioadenosine. J Biol Chem. 1982 Apr 25;257(8):4196–4202. [PubMed] [Google Scholar]
- Backlund P. S., Jr, Smith R. A. 5'-Methylthioadenosine metabolism and methionine synthesis in mammalian cells grown in culture. Biochem Biophys Res Commun. 1982 Sep 30;108(2):687–695. doi: 10.1016/0006-291x(82)90884-1. [DOI] [PubMed] [Google Scholar]
- Backlund P. S., Jr, Smith R. A. Methionine synthesis from 5'-methylthioadenosine in rat liver. J Biol Chem. 1981 Feb 25;256(4):1533–1535. [PubMed] [Google Scholar]
- Banerjee R. V., Matthews R. G. Cobalamin-dependent methionine synthase. FASEB J. 1990 Mar;4(5):1450–1459. doi: 10.1096/fasebj.4.5.2407589. [DOI] [PubMed] [Google Scholar]
- Bitonti A. J., McCann P. P., Sjoerdsma A. Restriction of bacterial growth by inhibition of polyamine biosynthesis by using monofluoromethylornithine, difluoromethylarginine and dicyclohexylammonium sulphate. Biochem J. 1982 Nov 15;208(2):435–441. doi: 10.1042/bj2080435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bushby S. R., Hitchings G. H. Trimethoprim, a sulphonamide potentiator. Br J Pharmacol Chemother. 1968 May;33(1):72–90. doi: 10.1111/j.1476-5381.1968.tb00475.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheung K. S., Wasserman S. A., Dudek E., Lerner S. A., Johnston M. Chloralanyl and propargylglycyl dipeptides. Suicide substrate containing antibacterials. J Med Chem. 1983 Dec;26(12):1733–1741. doi: 10.1021/jm00366a015. [DOI] [PubMed] [Google Scholar]
- Cooper A. J. Biochemistry of sulfur-containing amino acids. Annu Rev Biochem. 1983;52:187–222. doi: 10.1146/annurev.bi.52.070183.001155. [DOI] [PubMed] [Google Scholar]
- Ferro A. J., Barrett A., Shapiro S. K. 5-Methylthioribose kinase. A new enzyme involved in the formation of methionine from 5-methylthioribose. J Biol Chem. 1978 Sep 10;253(17):6021–6025. [PubMed] [Google Scholar]
- Fitchen J. H., Riscoe M. K., Ferro A. J. Exploitation of methylthioribose kinase in the development of antiprotozoal drugs. Adv Exp Med Biol. 1988;250:199–210. doi: 10.1007/978-1-4684-5637-0_18. [DOI] [PubMed] [Google Scholar]
- Furfine E. S., Abeles R. H. Intermediates in the conversion of 5'-S-methylthioadenosine to methionine in Klebsiella pneumoniae. J Biol Chem. 1988 Jul 15;263(20):9598–9606. [PubMed] [Google Scholar]
- Ghoda L. Y., Savarese T. M., Dexter D. L., Parks R. E., Jr, Trackman P. C., Abeles R. H. Characterization of a defect in the pathway for converting 5'-deoxy-5'-methylthioadenosine to methionine in a subline of a cultured heterogeneous human colon carcinoma. J Biol Chem. 1984 Jun 10;259(11):6715–6719. [PubMed] [Google Scholar]
- Gianotti A. J., Tower P. A., Sheley J. H., Conte P. A., Spiro C., Ferro A. J., Fitchen J. H., Riscoe M. K. Selective killing of Klebsiella pneumoniae by 5-trifluoromethylthioribose. Chemotherapeutic exploitation of the enzyme 5-methylthioribose kinase. J Biol Chem. 1990 Jan 15;265(2):831–837. [PubMed] [Google Scholar]
- Hewlett P. S. Measurement of the potencies of drug mixtures. Biometrics. 1969 Sep;25(3):477–487. [PubMed] [Google Scholar]
- Hitchings G. H. Folate antagonists as antibacterial and antiprotozoal agents. Ann N Y Acad Sci. 1971 Nov 30;186:444–451. doi: 10.1111/j.1749-6632.1971.tb31171.x. [DOI] [PubMed] [Google Scholar]
- Hitchings G. H. Trimethoprim: biochemical basis for sulphonamide potentiation. S Afr Med J. 1970 Aug 15;44(32 Suppl):1–2. [PubMed] [Google Scholar]
- Johnston M., Jankowski D., Marcotte P., Tanaka H., Esaki N., Soda K., Walsh C. Suicide inactivation of bacterial cystathionine gamma-synthase and methionine gamma-lyase during processing of L-propargylglycine. Biochemistry. 1979 Oct 16;18(21):4690–4701. doi: 10.1021/bi00588a033. [DOI] [PubMed] [Google Scholar]
- Kadurugamuwa J. L., Anwar H., Brown M. R., Zak O. Protein antigens of encapsulated Klebsiella pneumoniae surface exposed after growth in the presence of subinhibitory concentrations of cephalosporins. Antimicrob Agents Chemother. 1985 Aug;28(2):195–199. doi: 10.1128/aac.28.2.195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kerr D. S. O-acetylhomoserine sulfhydrylase from Neurospora. Purification and consideration of its function in homocysteine and methionine synthesis. J Biol Chem. 1971 Jan 10;246(1):95–102. [PubMed] [Google Scholar]
- Kredich N. M., Becker M. A., Tomkins G. M. Purification and characterization of cysteine synthetase, a bifunctional protein complex, from Salmonella typhimurium. J Biol Chem. 1969 May 10;244(9):2428–2439. [PubMed] [Google Scholar]
- Kredich N. M., Foote L. J., Hulanicka M. D. Studies on the mechanism of inhibition of Salmonella typhimurium by 1,2,4-triazole. J Biol Chem. 1975 Sep 25;250(18):7324–7331. [PubMed] [Google Scholar]
- Kredich N. M., Hulanicka M. D., Hallquist S. G. Synthesis of L-cysteine in Salmonella typhimurium. Ciba Found Symp. 1979;(72):87–99. doi: 10.1002/9780470720554.ch6. [DOI] [PubMed] [Google Scholar]
- Pegg A. E., Williams-Ashman H. G. Phosphate-stimulated breakdown of 5'-methylthioadenosine by rat ventral prostate. Biochem J. 1969 Nov;115(2):241–247. doi: 10.1042/bj1150241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Riscoe M. K., Ferro A. J., Fitchen J. H. Analogs of 5-methylthioribose, a novel class of antiprotozoal agents. Antimicrob Agents Chemother. 1988 Dec;32(12):1904–1906. doi: 10.1128/aac.32.12.1904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Riscoe M. K., Ferro A. J., Fitchen J. H. Methionine recycling as a target for antiprotozoal drug development. Parasitol Today. 1989 Oct;5(10):330–333. doi: 10.1016/0169-4758(89)90128-2. [DOI] [PubMed] [Google Scholar]
- Schlenk F. Methylthioadenosine. Adv Enzymol Relat Areas Mol Biol. 1983;54:195–265. doi: 10.1002/9780470122990.ch4. [DOI] [PubMed] [Google Scholar]
- Shapiro S. K., Barrett A. 5-Methylthioribose as a precursor of the carbon chain of methionine. Biochem Biophys Res Commun. 1981 Sep 16;102(1):302–307. doi: 10.1016/0006-291x(81)91521-7. [DOI] [PubMed] [Google Scholar]
- Shapiro S. K., Schlenk F. Conversion of 5'-methylthioadenosine into S-adenosylmethionine by yeast cells. Biochim Biophys Acta. 1980 Dec 1;633(2):176–180. doi: 10.1016/0304-4165(80)90403-1. [DOI] [PubMed] [Google Scholar]
- Trackman P. C., Abeles R. H. Methionine synthesis from 5'-S-Methylthioadenosine. Resolution of enzyme activities and identification of 1-phospho-5-S methylthioribulose. J Biol Chem. 1983 Jun 10;258(11):6717–6720. [PubMed] [Google Scholar]
- Williams-Ashman H. G., Seidenfeld J., Galletti P. Trends in the biochemical pharmacology of 5'-deoxy-5'-methylthioadenosine. Biochem Pharmacol. 1982 Feb 1;31(3):277–288. doi: 10.1016/0006-2952(82)90171-x. [DOI] [PubMed] [Google Scholar]


