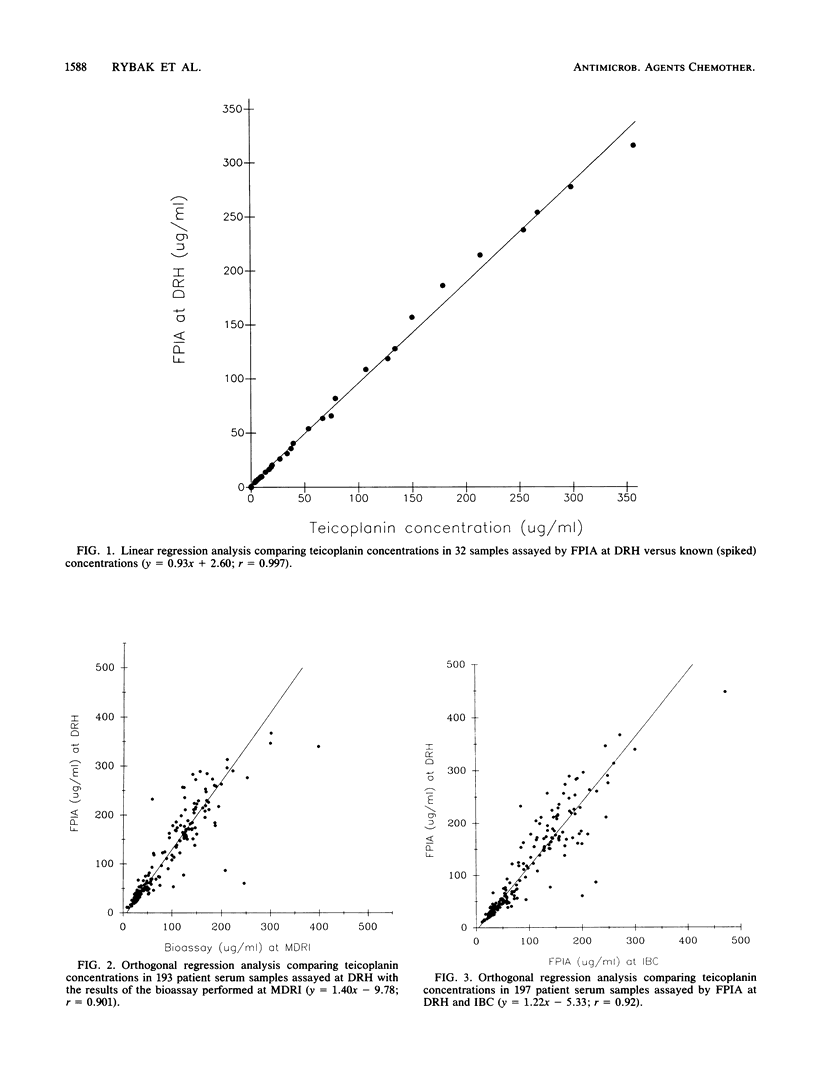
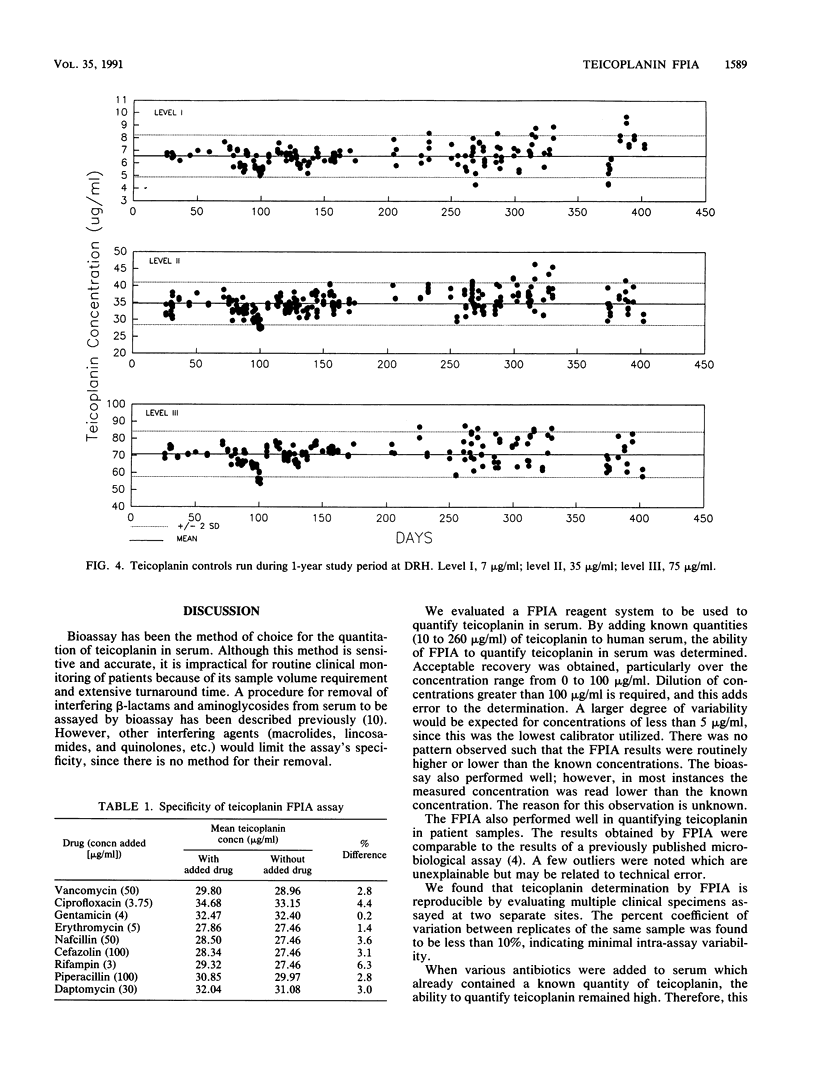
Abstract
A teicoplanin fluorescence polarization immunoassay (FPIA) developed by International BioClinical (IBC) was evaluated by using serum samples from patients who had been receiving teicoplanin at Detroit Receiving Hospital (DRH) as part of a clinical investigation. Patient samples collected over a 1-year span were assayed at DRH and at IBC, and the results were compared with those of a standard microbiological assay performed at Merrell Dow Research Institute, Indianapolis, Ind. The FPIA has a rapid turnaround time (circa 20 min), utilizes small sample volumes (less than 100 microliters) and is sensitive and accurate in determining concentrations in the range of 5 to 100 micrograms/ml. The intra-assay and interassay coefficient of variation for controls (7, 35, and 75 micrograms/ml) was less than or equal to 13%. Concentrations greater than 100 micrograms/ml must be diluted prior to the assay, which may introduce additional error in determination. The FPIA compared well with the bioassay (r = 0.901) for 193 clinical samples. The results obtained utilizing the FPIA system were reproducible at two different sites, as illustrated by the high degree of correlation between the results at DRH and IBC (r = 0.92). There was less than 7% interference noted when teicoplanin was assayed in the presence of other antibiotics. Patient samples stored for up to 1 year retained their potency: the mean recovery rate in these samples was 107%. The FPIA should be useful for monitoring and adjusting teicoplanin dosage regimens in patients.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bibler M. R., Frame P. T., Hagler D. N., Bode R. B., Staneck J. L., Thamlikitkul V., Harris J. E., Haregewoin A., Bullock W. E., Jr Clinical evaluation of efficacy, pharmacokinetics, and safety of teicoplanin for serious gram-positive infections. Antimicrob Agents Chemother. 1987 Feb;31(2):207–212. doi: 10.1128/aac.31.2.207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chambers H. F., Kennedy S. Effects of dosage, peak and trough concentrations in serum, protein binding, and bactericidal rate on efficacy of teicoplanin in a rabbit model of endocarditis. Antimicrob Agents Chemother. 1990 Apr;34(4):510–514. doi: 10.1128/aac.34.4.510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Erickson R. C., Hildebrand A. R., Hoffman P. F., Gibson C. B. A sensitive bioassay for teicoplanin in serum in the presence or absence of other antibiotics. Diagn Microbiol Infect Dis. 1989 May-Jun;12(3):235–241. doi: 10.1016/0732-8893(89)90020-5. [DOI] [PubMed] [Google Scholar]
- Gilbert D. N., Wood C. A., Kimbrough R. C. Failure of treatment with teicoplanin at 6 milligrams/kilogram/day in patients with Staphylococcus aureus intravascular infection. The Infectious Diseases Consortium of Oregon. Antimicrob Agents Chemother. 1991 Jan;35(1):79–87. doi: 10.1128/aac.35.1.79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glupczynski Y., Lagast H., Van der Auwera P., Thys J. P., Crokaert F., Yourassowsky E., Meunier-Carpentier F., Klastersky J., Kains J. P., Serruys-Schoutens E. Clinical evaluation of teicoplanin for therapy of severe infections caused by gram-positive bacteria. Antimicrob Agents Chemother. 1986 Jan;29(1):52–57. doi: 10.1128/aac.29.1.52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jehl F., Monteil H., Tarral A. HPLC quantitation of the six main components of teicoplanin in biological fluids. J Antimicrob Chemother. 1988 Jan;21 (Suppl A):53–59. doi: 10.1093/jac/21.suppl_a.53. [DOI] [PubMed] [Google Scholar]
- Jolley M. E. Fluorescence polarization immunoassay for the determination of therapeutic drug levels in human plasma. J Anal Toxicol. 1981 Sep-Oct;5(5):236–240. doi: 10.1093/jat/5.5.236. [DOI] [PubMed] [Google Scholar]
- Joos B., Lüthy R. Determination of teicoplanin concentrations in serum by high-pressure liquid chromatography. Antimicrob Agents Chemother. 1987 Aug;31(8):1222–1224. doi: 10.1128/aac.31.8.1222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kenny M. T., Dulworth J. K., Brackman M. A., Torney H. L., Gibson C. B., Hildebrand A. R., Weckbach L. S., Staneck J. L. Bioassay of teicoplanin in serum containing rifampin or a beta-lactam antibiotic. Diagn Microbiol Infect Dis. 1989 Sep-Oct;12(5):449–454. doi: 10.1016/0732-8893(89)90119-3. [DOI] [PubMed] [Google Scholar]
- Rybak M. J., Lerner S. A., Levine D. P., Albrecht L. M., McNeil P. L., Thompson G. A., Kenny M. T., Yuh L. Teicoplanin pharmacokinetics in intravenous drug abusers being treated for bacterial endocarditis. Antimicrob Agents Chemother. 1991 Apr;35(4):696–700. doi: 10.1128/aac.35.4.696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verbist L., Tjandramaga B., Hendrickx B., Van Hecken A., Van Melle P., Verbesselt R., Verhaegen J., De Schepper P. J. In vitro activity and human pharmacokinetics of teicoplanin. Antimicrob Agents Chemother. 1984 Dec;26(6):881–886. doi: 10.1128/aac.26.6.881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams A. H., Grüneberg R. N., Webster A., Ridgway G. L. Teicoplanin in the treatment of infection caused by gram-positive organisms. J Hosp Infect. 1986 Mar;7 (Suppl A):101–103. doi: 10.1016/0195-6701(86)90014-9. [DOI] [PubMed] [Google Scholar]


