Abstract
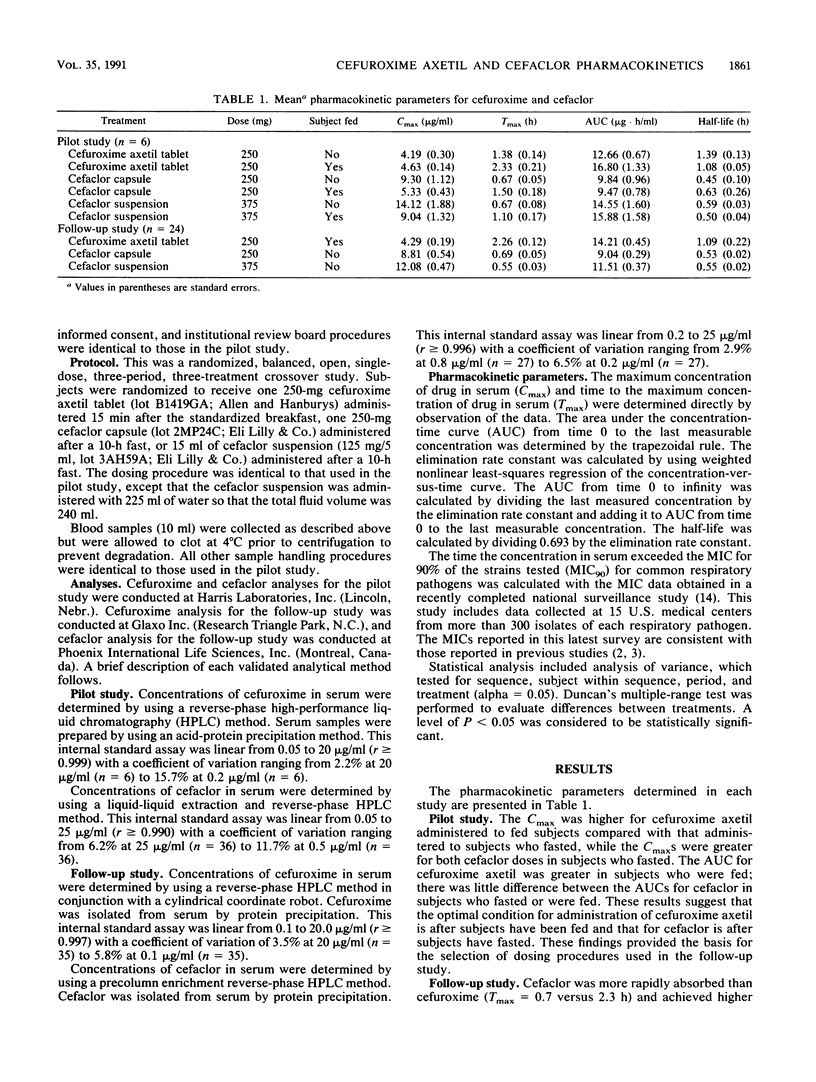
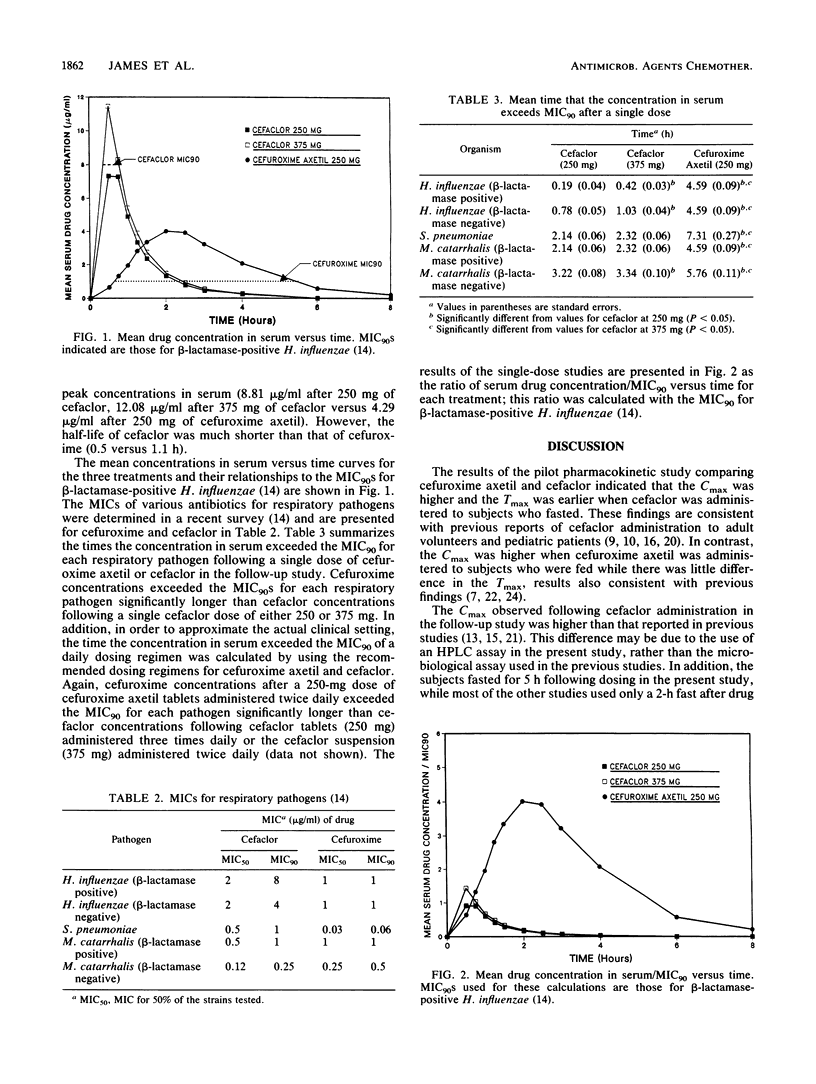
The pharmacokinetics of single doses of cefaclor at 250 and 375 mg and cefuroxime axetil at 250 mg administered under optimal conditions (i.e., cefuroxime axetil after food and cefaclor in the fasted state) were studied in 24 healthy male volunteers. Drug concentrations in serum were related to MICs for common respiratory tract pathogens by using data generated from a recently completed national survey. The time the concentrations in serum exceeded the MICs for Haemophilus influenzae, Streptococcus pneumoniae, and Moraxella (formerly Branhamella) catarrhalis were significantly greater (P less than 0.05) for cefuroxime axetil at 250 mg than for cefaclor at 250 or 375 mg. With the recommended dosing regimens (cefuroxime axetil at 250 mg and cefaclor at 375 mg twice daily or cefaclor at 250 mg three times daily), cefuroxime concentrations exceed the MIC for 90% of the strains tested for a greater time period than cefaclor concentrations with either regimen. The reasons for this difference are (i) the greater potency and slower clearance of cefuroxime compared with those of cefaclor and (ii) the greater sensitivity of these pathogens to cefuroxime.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Doern G. V., Jones R. N. Antimicrobial susceptibility testing of Haemophilus influenzae, Branhamella catarrhalis, and Neisseria gonorrhoeae. Antimicrob Agents Chemother. 1988 Dec;32(12):1747–1753. doi: 10.1128/aac.32.12.1747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doern G. V., Jorgensen J. H., Thornsberry C., Preston D. A., Tubert T., Redding J. S., Maher L. A. National collaborative study of the prevalence of antimicrobial resistance among clinical isolates of Haemophilus influenzae. Antimicrob Agents Chemother. 1988 Feb;32(2):180–185. doi: 10.1128/aac.32.2.180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drusano G. L. Role of pharmacokinetics in the outcome of infections. Antimicrob Agents Chemother. 1988 Mar;32(3):289–297. doi: 10.1128/aac.32.3.289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drusano G. L., Ryan P. A., Standiford H. C., Moody M. R., Schimpff S. C. Integration of selected pharmacologic and microbiologic properties of three new beta-lactam antibiotics: a hypothesis for rational comparison. Rev Infect Dis. 1984 May-Jun;6(3):357–363. doi: 10.1093/clinids/6.3.357. [DOI] [PubMed] [Google Scholar]
- Finn A., Straughn A., Meyer M., Chubb J. Effect of dose and food on the bioavailability of cefuroxime axetil. Biopharm Drug Dispos. 1987 Nov-Dec;8(6):519–526. doi: 10.1002/bdd.2510080604. [DOI] [PubMed] [Google Scholar]
- Foord R. D. Cefuroxime: human pharmacokinetics.. Antimicrob Agents Chemother. 1976 May;9(5):741–747. doi: 10.1128/aac.9.5.741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ginsburg C. M. Comparative pharmacokinetics of cefadroxil, cefaclor, cephalexin and cephradine in infants and children. J Antimicrob Chemother. 1982 Sep;10 (Suppl B):27–31. doi: 10.1093/jac/10.suppl_b.27. [DOI] [PubMed] [Google Scholar]
- Glynne A., Goulbourn R. A., Ryden R. A human pharmacology study of cefaclor. J Antimicrob Chemother. 1978 Jul;4(4):343–348. doi: 10.1093/jac/4.4.343. [DOI] [PubMed] [Google Scholar]
- Hampel B., Lode H., Wagner J., Koeppe P. Pharmacokinetics of cefadroxil and cefaclor during an eight-day dosage period. Antimicrob Agents Chemother. 1982 Dec;22(6):1061–1063. doi: 10.1128/aac.22.6.1061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harding S. M., Williams P. E., Ayrton J. Pharmacology of Cefuroxime as the 1-acetoxyethyl ester in volunteers. Antimicrob Agents Chemother. 1984 Jan;25(1):78–82. doi: 10.1128/aac.25.1.78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hodges G. R., Liu C., Hinthorn D. R., Harms J. L., Dworzack D. L. Pharmacological evaluation of cefaclor in volunteers. Antimicrob Agents Chemother. 1978 Sep;14(3):454–456. doi: 10.1128/aac.14.3.454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jorgensen J. H., Doern G. V., Maher L. A., Howell A. W., Redding J. S. Antimicrobial resistance among respiratory isolates of Haemophilus influenzae, Moraxella catarrhalis, and Streptococcus pneumoniae in the United States. Antimicrob Agents Chemother. 1990 Nov;34(11):2075–2080. doi: 10.1128/aac.34.11.2075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Korzeniowski O. M., Scheld W. M., Sande M. A. Comparative pharmacology of cefaclor and cephalexin. Antimicrob Agents Chemother. 1977 Aug;12(2):157–162. doi: 10.1128/aac.12.2.157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCracken G. H., Jr, Ginsburg C. M., Clahsen J. C., Thomas M. L. Pharmacokinetics of cefaclor in infants and children. J Antimicrob Chemother. 1978 Nov;4(6):515–521. doi: 10.1093/jac/4.6.515. [DOI] [PubMed] [Google Scholar]
- Nightingale C. H. Interaction of microbiology and pharmacokinetics in the selection of appropriate antibiotic therapy. DICP. 1989 Jul-Aug;23(7-8):S16–S20. doi: 10.1177/106002808902300704. [DOI] [PubMed] [Google Scholar]
- Nightingale C. Pharmacokinetics of the oral cephalosporins in adults. J Int Med Res. 1980;8(Suppl 1):2–8. [PubMed] [Google Scholar]
- Shanker S. A., Baird-Lamber J., Cvejic M., Buchanan N. Effect of food on cefaclor bioavailability in children. Med J Aust. 1983 Oct 15;2(8):372–372. doi: 10.5694/j.1326-5377.1983.tb122529.x. [DOI] [PubMed] [Google Scholar]
- Sides G. D., Franson T. R., DeSante K. A., Black H. R. A comprehensive review of the clinical pharmacology and pharmacokinetics of cefaclor. Clin Ther. 1988;11 (Suppl A):5–19. [PubMed] [Google Scholar]
- Sommers D. K., van Wyk M., Moncrieff J., Schoeman H. S. Influence of food and reduced gastric acidity on the bioavailability of bacampicillin and cefuroxime axetil. Br J Clin Pharmacol. 1984 Oct;18(4):535–539. doi: 10.1111/j.1365-2125.1984.tb02501.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tally F. P., Jacobus N. V., Barza M. In vitro activity and serum protein-binding of cefaclor. J Antimicrob Chemother. 1979 Mar;5(2):159–165. doi: 10.1093/jac/5.2.159. [DOI] [PubMed] [Google Scholar]
- Williams P. E., Harding S. M. The absolute bioavailability of oral cefuroxime axetil in male and female volunteers after fasting and after food. J Antimicrob Chemother. 1984 Feb;13(2):191–196. doi: 10.1093/jac/13.2.191. [DOI] [PubMed] [Google Scholar]


