Abstract
cAMP is a major regulator of CRH transcription. However, receptors activating CRH neurons (α-adrenergic and glutamatergic) do not signal through cAMP, suggesting that calcium phospholipid-dependent signaling synergizes with small elevations of intracellular cAMP. To test this hypothesis, we examined the relationship between activation of CRH transcription, cAMP production, and cAMP response element binding protein (CREB) phosphorylation in neuronal cultures treated with the adenylyl cyclase stimulator, forskolin, the phorbol ester, phorbol-12-myristate-13-acetate (PMA), or their combination. Forskolin, at threshold concentrations for cAMP production and CREB phosphorylation, induced CRH promoter-driven luciferase activity in 4B cells (EC50 = 0.7 μm) and CRH primary transcript in hypothalamic neurons (EC50 = 0.6 μm). PMA alone failed to activate CRH transcription despite being as effective as forskolin in phosphorylating CREB (Ser133 and Ser121). Although PMA potentiated the effect of low forskolin concentrations on CRH transcription and CREB phosphorylation, there was no correlation between phosphorylated CREB levels and activation of CRH transcription. Similarly, the calcium/calmodulin-dependent kinase inhibitor, KN-93, enhanced PMA plus forskolin-stimulated CREB phosphorylation and inhibited CRH transcription. Suppression of CREB phosphorylation by the protein kinase A inhibitor, H89, or the CREB dominant negative, A-CREB, did not affect basal but blocked forskolin-stimulated transcription. This study shows that calcium phospholipid-dependent pathways potentiate the ability of small elevations of intracellular cAMP to activate CRH transcription, providing a mechanism by which non-cAMP-dependent regulators induce CRH gene expression. In addition, the data indicate that phosphorylated CREB is essential but not sufficient for activation of CRH transcription, suggesting that full promoter stimulation requires the interaction of phosphorylated CREB with a coactivator.
CRH IS A MAJOR neuropeptide controlling behavioral and autonomic responses to stress. Given a stressful stimulus, CRH is rapidly released from neuronal terminals of parvocellular neurons of the hypothalamic paraventricular nucleus located in the external zone of the median eminence into the pituitary portal circulation (1,2). This leads to consecutive release of pituitary adrenocorticotropic hormone and adrenocortical glucocorticoids, the latter being essential for metabolic and behavioral adaptation to stress (3). Activation of the hypothalamus-pituitary-adrenal axis is usually associated with rapid and transient increases in CRH transcription, which are necessary to replenish CRH mRNA and peptide levels after its release (4). Dysregulation of CRH has been implicated in a number of disorders, such as depression, anxiety, and posttraumatic stress disorder. This could result from alterations in circulating glucocorticoids secondary to CRH-dependent regulation of hypothalamus-pituitary-adrenal axis activity as well as from increased expression of CRH itself, acting as a neurotransmitter on receptors located in limbic areas of the brain (4,5). Therefore, elucidation of the mechanisms controlling CRH transcription is essential for a better understanding of the pathophysiology and treatment of stress-related disorders.
It is well established that cAMP can induce CRH expression through activation of protein kinase A (PKA) and binding of phosphorylated (phospho) cAMP response element binding protein (CREB) to the cAMP response element (CRE) (6,7,8). Furthermore, Nikodemova et al. (9) have shown that only minor increases in cAMP are sufficient to induce maximal increases in CRH promoter activity. Whereas it is clear that cAMP and the CRE play an integral role in the regulation of CRH gene transcription, cAMP is not the major signaling pathway of most neurotransmitters believed to directly initiate activation of the CRH neuron, namely glutamate and norepinephrine (10,11). Although norepinephrine can activate β-adrenergic receptors with lower affinity, in vivo and in vitro evidence suggest that α- but not β-adrenergic receptors mediate the direct stimulatory effect of norepinephrine in CRH neurons (12,13,14). In situ hybridization studies using receptor subtype specific probes have shown that the subtype present in CRH neurons is the α1-adrenergic receptor (15,16,17). Whereas this receptor subtype is coupled to phospholipase C and stimulation of protein kinase C and increases in cytosolic calcium, it can also stimulate low levels of cAMP production (18). Thus, it is possible that stimulation of calcium/phospholipid signaling by glutamate and norepinephrine facilitates the stimulatory effect of small increases in cAMP, leading to full activation of the CRH neuron. In the present study, we used the hypothalamic neuronal cell line, 4B, as well as primary cultures of rat hypothalamic neurons to investigate the interaction between cAMP-dependent and -independent signaling on the regulation of CRH transcription.
Materials and Methods
Constructs and reagents
The CRH promoter-driven luciferase reporter gene (pCRH-Luc) was created by two step cloning, using a 613-bp restriction fragment containing the CRH promoter (−498 to +115 bp relative to the proximal transcription start point), obtained by XbaI/KpnI digestion of the CRH gene clone prCRHBglII (provided by Dr. Audrey Seasholtz, University of Michigan, Ann Arbor, MI). The DNA fragment was cloned into the XbaI/KpnI sites of pcDNA3.1(−) (Invitrogen, San Diego, CA), and then it was subcloned into the NheI-HindIII sites of pGL3Basic (Promega Corp., Madison, WI) (3). The dominant-negative bZIP expression vector, A-CREB, was provided by Dr. Charles Vinson (National Cancer Institute, Bethesda, MD).
Cell cultures, transfection, and treatments
4B cells, provided by Dr. John Kasckow (University of Cincinnati College of Medicine, Cincinnati, OH), were cultured in DMEM (Invitrogen) and supplemented with 10% fetal bovine serum, 10% horse serum, and 100 U/ml penicillin and 100 μg/ml streptomycin (19). For reporter gene assays, cells were transfected by electroporation using the Nucleofector (Amaxa, Gaithersburg, MD) protocol and solution V purchased from the manufacturer. Three million cells were transfected with 5 μg of CRHp-luc plasmid and 75 ng of renilla luciferase construct to normalize for transfection efficiency. After transfection, cells were resuspended in growth medium and plated into 48-well culture plates. Sixteen to 18 h after transfection, cells were changed into serum-free medium containing 0.1% BSA and 1 h later incubated for 6 h with forskolin (Sigma, St. Louis, MO), the phorbol ester 12-myristate 13-acetate (PMA; Sigma), the PKA inhibitor H89 (BIOMOL, Plymouth Meeting, PA), or the calcium calmodulin-dependent kinase inhibitor, KN-93 (BIOMOL) in the conditions described in results and figure legends. Cells were then washed in PBS and lysed in 100 μl of passive lysis buffer. Luciferase activity in cell lysates was determined using reagents from Promega (dual luciferase assay system).
Primary culture of hypothalamic cells
Fetal Sprague Dawley rats, embryonic d 18, were used to obtain primary hypothalamic neurons for culture. Fetal rats were rapidly removed from 18-d pregnant rats after CO2 sedation and decapitation. Fetuses were decapitated and hypothalamic tissue dissected and collected in ice-cold buffer (pH 7.4, containing 137 mm NaCl, 5 mm KCl, 0.7 mm Na2HPO4, 25 mm HEPES buffer, and 100 μg/ml gentamicin). Tissues were then digested for 1.5 h with collagenase type 2 (1 mg/ml; Worthington, Lakewood, NJ) dissolved in the above buffer, supplemented with 1 mg/ml glucose, 4 mg/ml BSA, and 0.2 mg/ml deoxyribonuclease. The cell suspension was filtered through a 40-μm cell strainer (BD Falcon, San Jose, CA) centrifuged at 200 × g for 10 min. Cells were consecutively washed twice in dispersion buffer and once in plating media (DMEM/F12, 100 μg/ml gentamicin, and 10% heat-inactivated fetal bovine serum). Cells were plated at a density of 1 × 106 cells/well in six-well plates coated with poly-l-lysine. Media were changed to Neurobasal Media (Life Technologies, Inc./Invitrogen, Carlsbad, CA) with B27 supplement (Invitrogen), 0.5 mm l-glutamate, and 100 μg/ml gentamicin on the second day to support neuron growth. From d 5 to 9 (i.e. for 5 d) cells were cultured in the presence of 5 μm cytosine arabinoside (Sigma), a selective inhibitor of DNA synthesis, which was added to the neurobasal media to prevent glial proliferation. On d 10, cells were changed into serum-free/B27 supplement-free neurobasal medium containing 0.1% BSA for 4 h before treatment in the conditions described in results and figure legends.
Immunoblotting
Nuclear extracts from 4B cells were prepared using NE-PER nuclear and cytoplasmic extraction reagent (Pierce, Rockford, IL) according to the manufacturer’s protocol. Protein concentration was quantified by spectrophotometry using the BCA protein assay (Pierce). For Western blot, 15 μg of nuclear extract were loaded and separated in a 10% Tris-glycine gel (Invitrogen). Proteins were transferred to a polyvinyl difluoride membrane (GE Amersham Biosciences, Piscataway, NJ), incubated with 5% nonfat milk in 1× Tris-buffered saline plus 0.05% Tween 20 for 1 h and incubated overnight at 4 C with anti-phospho-CREB antibody (Upstate Biotechnology, Lake Placid, NY), at a 1:3000 dilution. After washing in 1× Tris-buffered saline plus 0.05% Tween 20, membranes were incubated for 1 h at room temperature with a horseradish peroxidase-conjugated donkey antirabbit IgG at a dilution of 1:20,000. Detection of immunoreactive bands was performed using ECL Plus TM reagents (GE Amersham Biosciences) followed by exposure to BioMax MR film (Eastman Kodak, Rochester, NY). After film exposure, blots were stripped and assayed for transcription intermediary factor-2B or specificity protein-1 as a loading control. The intensity of the bands was quantified using a computer image analysis system, ImageJ (developed at the National Institutes of Health, which is freely available at http://rsb.info.nih.gov/).
Measurement of intracellular cAMP
4B cells and primary hypothalamic neurons were plated in 12-well plates at a density of 5 × 105 cells/well. Cells were treated with increasing concentrations of forskolin with or without the addition of 1 μm PMA, in the presence of 1 mm isobutyl-methylxanthine (IBMX; Sigma), for 20 min. After treatment, cells were washed twice with cold PBS, and intracellular cAMP was extracted by addition of 500 μl of 0.1 n HCl with 0.1% Triton X-100, followed by three cycles of freezing and thawing. cAMP concentration was measured by RIA (PerkinElmer, Norwalk, CT) (9).
RNA isolation
After treatments, primary neurons were harvested in 1 ml of TRIzol reagent (Invitrogen), transferred to 1.5 ml Microfuge tubes, and centrifuged at 11,000 × g for 15 min after addition of 0.2 ml chloroform. The RNA-containing upper aqueous phase was removed, and RNA was precipitated with an equal amount of 70% ethanol and then transferred to an RNeasy spin column (QIAGEN, Valencia, CA). RNA was then purified using the RNeasy minikit (QIAGEN) according to the manufacturer’s protocol with on-column deoxyribonuclease (QIAGEN) digestion to eliminate genomic contamination. Total RNA concentration was measured by Nanodrop spectrophotometry.
Real-time PCR for CRH heteronuclear (hn) RNA
CRH primary transcript levels (CRH hnRNA) were measured by quantitative RT-PCR (qRT-PCR), using the LUX gene expression system (Invitrogen). LUX fluorogenic and unlabeled primer pairs were designed using the D-LUX designer software (Invitrogen) by targeting the intron of the CRH gene. The sequence of FAM-labeled forward primer is CACAGGCGGCGAATAGCTTAA-ACCTG (FAM)G, and the unlabeled reverse primer is CAGGTGACCCTTCCTTGGAGA. Platinum Quantitative PCR SuperMix-UDG (Invitrogen) was used for the amplification mixture with each LUX primer at a final concentration of 200 nm and 2 μl of cDNA for a total reaction volume of 25 μl. PCRs were performed on spectrofluorometric thermal cycler (ABI PRISM 7300 real time PCR system; Applied Biosystems, Foster City, CA). Samples were amplified by an initial denaturation at 50 C for 2 min and 95 C for 2 min and then cycled (45 times) using 95 C for 15 sec, 57 C for 30 sec, and 60 C for 15 sec. Calibration curves to assess the efficiency of the PCRs were performed using a 5.5-kb BglII rat genomic fragment containing the full rat CRH gene (−498 to 4969) cloned into pUC18, kindly provided by Dr. Audrey Seasholtz.
For the experimental samples, 550 ng of total RNA per sample were used for reverse transcription into cDNA. RNA was primed with oligo(dT)20, and cDNA was synthesized using SuperScript III first-strand synthesis system for RT-PCR (Invitrogen). PCRs with CRH intronic primers were performed in parallel with glyceraldehyde 3-phosphate dehydrogenase (GAPDH) primers, used as control gene. Predesigned Joe-labeled forward and unlabeled reverse GAPDH primers were obtained from Invitrogen. Relative quantification of CRH hnRNA levels was performed using the comparative threshold cycle method for CRH hnRNA and GAPDH, as described in the user’s manual (20). The absence of RNA detection when the reverse transcription step was omitted indicated the lack of genomic DNA contamination in the RNA samples.
Statistical analysis
Statistical significance of the differences between groups was calculated by one- or two-way ANOVA, followed by Student-Newman-Keuls method for pair-wise multiple comparisons. Statistical significance was set at P < 0.05. Data are presented as means ± sem from the values in the number of observations indicated in results or legends to figures.
Results
PMA potentiates the effect of forskolin on CRH promoter activity in 4B cells
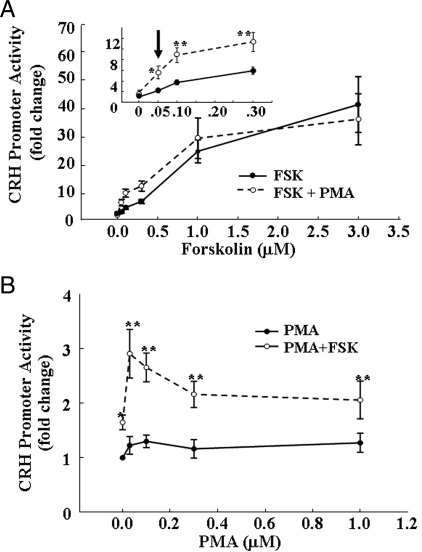
To study the interaction between cAMP-dependent and independent pathways on CRH transcription we examined the effects of the adenylyl cyclase stimulant, forskolin, and the phorbol ester, PMA, on CRH promoter-driven luciferase activity. Treatment of 4B cells transfected with pCRH-Luc with forskolin at concentrations from 0.05 to 3 μm resulted in a concentration-dependent increase in luciferase activity, with an EC50 of 0.7 μm and a maximal stimulatory concentration of 3 μm forskolin. Incubation of the cells with 1 μm PMA alone had no effect on CRH promoter activity but it potentiated the effect of low concentrations of forskolin, decreasing the EC50 to 0.3 μm (Fig. 1A). The positive interaction between low concentrations of forskolin and PMA was also shown when cells were incubated with 0.1 μm forskolin and increasing concentrations of PMA. As shown in Fig. 1B, PMA alone failed to stimulate CRH promoter activity at any concentration used (0.05–1 μm). Forskolin (0.1 μm) alone caused a small 1.6-fold increase in CRH promoter activity, but when added together with low concentrations of PMA (0.03 μm), there was a significantly higher increase of 2.9-fold, P < 0.001, compared with forskolin alone. Increasing the concentration of PMA reduced the potentiating effect on forskolin-stimulated CRH promoter activity; with PMA concentrations higher than 0.1 μm, CRH promoter activity was similar to that induced by forskolin alone (Fig. 1B).
Figure 1.
Synergistic actions of the adenylyl cyclase stimulator, forskolin, and the phorbol ester, PMA, on CRH promoter activity. Cultured 4B cells transiently transfected with pCRH-Luc and incubated for 6 h either with increasing concentrations of forskolin in the presence and absence of 1 μm PMA (A) or with increasing concentrations of PMA in the presence or absence of 0.1 μm forskolin (B) before measurement of luciferase activity. The inset (A) depicts the changes at the low forskolin concentrations shown within the square. Data points are the mean and se of values obtained in four experiments. *, P < 0.01 vs. respective concentrations of forskolin or PMA alone; **, P < 0.001 vs. forskolin or PMA alone.
Measurement of CRH primary transcript by intronic qRT-PCR
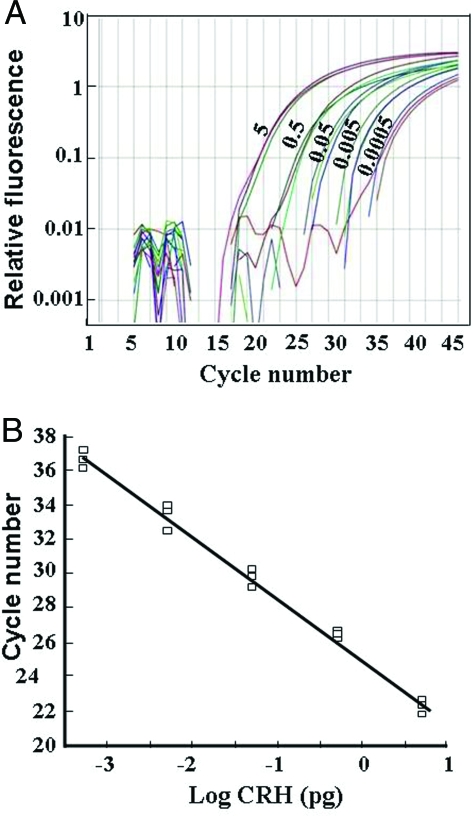
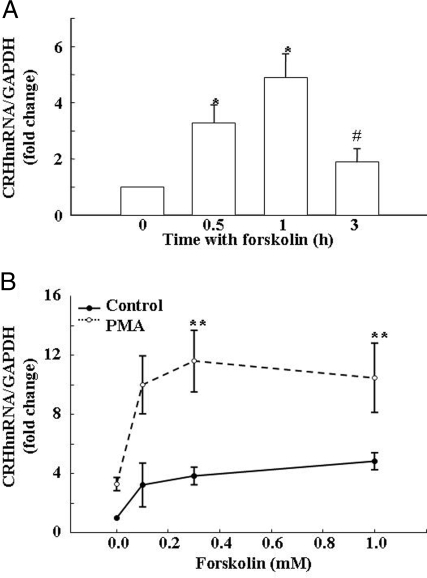
Standard curves for the effect of increasing the amounts of DNA template on product generation revealed a linear relationship using 0.5 fg to 5 pg of DNA (Fig. 2, A and B). Readings within the linear range of the curve were obtained using RNA from primary rat hypothalamic cell, whereas detection was at the background level using RNA prepared from the hypothalamic cell line H32. Levels of CRH hnRNA in primary hypothalamic cell cultures in basal conditions were very low, detectable in between 35 and 36 cycles. These levels increased to a maximum of about 5-fold after incubation of the cells with 10 μm forskolin. The time course of the effect of forskolin on CRH hnRNA in primary cultures of hypothalamic cells is shown in Fig. 3A. Incubation of the cells with forskolin resulted in a progressive increase in CRH hnRNA, reaching a maximum of about 6-fold by 1 h. The effect of forskolin was transient, declining to near basal levels by 3 h (Fig. 3A). Based on this time course, CRH hnRNA measurements were performed at 45 min in all consecutive experiments.
Figure 2.
Measurement of CRH primary transcript by intronic qRT-PCR. A, Fluorescence curves for intronic CRH RNA using the concentrations of a plasmid containing exonic and intronic sequences of the CRH gene indicated next to each curve (0. 5–5000 fg). B, Standard curve generated from data shown in A. All determinations were performed in triplicate. In RNA samples from hypothalamic cells cultures, CRH hnRNA was detectable with 35–36 cycles in basal conditions and 30–34 cycles under stimulated conditions. No signal over background levels was observed using RNA extracted from the hypothalamic cell line H32, which does not express CRH.
Figure 3.
Effects of forskolin and PMA on CRH hnRNA levels in primary cultures of hypothalamic cells. A, Time course of the effects of forskolin on CRH hnRNA in primary hypothalamic cultures. Bars are the mean and se of the results of four experiments, each performed in duplicate incubations and triplicate qRT-PCR. *, P < 0.01 vs. basal; #, P < 0.01 vs. 0.5 and 1 h. B, Synergistic action of forskolin and PMA on CRH hnRNA production in hypothalamic cell cultures. Cells were incubated for 45 min with increasing concentrations of forskolin in the presence and absence of PMA before measurement of CRH hnRNA using qRT-PCR. Data are the mean and se of values obtained in three to 11 experiments per point. Each experiment was performed in duplicate incubations and triplicate qRT-PCR. *, P < 0.05 vs. basal; **, P < 0.001.
PMA potentiates forskolin-stimulated CRH hnRNA production in primary cultures of hypothalamic neurons
To determine whether the synergistic effects of forskolin and PMA on CRH promoter activity occur in nontransformed CRH neurons, we used qRT-PCR to examine the ability of these compounds to stimulate CRH primary transcript (hnRNA) in primary cultures of hypothalamic neurons. The stimulatory effect of forskolin on CRH hnRNA was concentration dependent, with an EC50 of 0.14 μm and maximal stimulation of 4.8-fold the basal values (P < 0.05), with 0.5 μm forskolin (Fig. 3B). As in 4B cells, PMA alone had a minor and not significant effect on CRH hnRNA but it markedly potentiated the effect of forskolin, doubling the maximal stimulation by forskolin alone (P < 0.01), and decreasing the EC50 to 0.6 μm forskolin (P < 0.001) (Fig. 3B).
Interaction of forskolin and PMA on cAMP accumulation
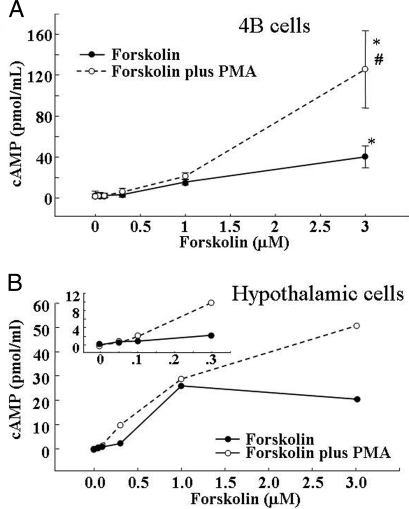
To determine whether the effect of PMA on forskolin-stimulated CRH promoter activity was due to a potentiation of the ability of forskolin to induce cAMP, we measured intracellular cAMP accumulation in the presence of the phosphodiesterase inhibitor, IBMX. As shown in Fig. 4, incubation of 4B cells with forskolin caused concentration-dependent increases in intracellular cAMP accumulation, with significant increases only at the high concentration of forskolin used, 3 μm (P < 0.001). PMA alone (1 μm) had no effect on cAMP production but significantly potentiated the effect of high concentrations of forskolin. The stimulatory effect of low concentrations of forskolin on intracellular cAMP production was slightly but not significantly affected by PMA (Fig. 4A).
Figure 4.
Dose response for the effect of forskolin and forskolin plus PMA on cAMP production in 4B cells (A) and primary cultures of hypothalamic cells (B). Cells were incubated for 20 min with 1 mm IBMX and increasing concentrations of forskolin in the presence and the absence of 1 μm PMA. In both systems, PMA potentiated the stimulatory effect of high concentrations of forskolin on cAMP production.
As in 4B cells, PMA on its own had no effect on cAMP production in hypothalamic neuronal cultures, but it potentiated the stimulatory effect forskolin on cAMP production (Fig. 4B). However, in primary hypothalamic cultures, the potentiating effect of PMA was evident at lower concentrations of forskolin than in 4Bcells (0.3 μm), at which PMA effectively potentiated the stimulatory effect of forskolin on CRH hnRNA levels (Fig. 4B).
Interaction between forskolin and PMA on CREB phosphorylation
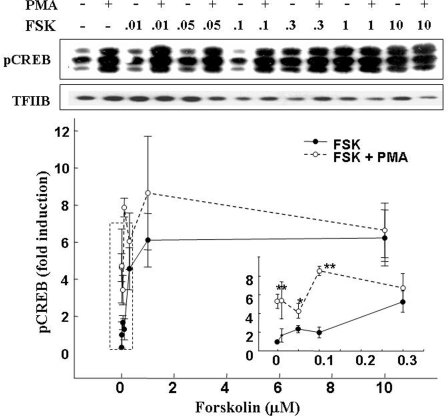
The concentration-response curve for CREB phosphorylation after incubation of 4B cells with forskolin, revealed marked increases in Ser 133-phospho-CREB, with a threshold concentration of 0.3 μm forskolin, a maximal stimulatory concentration of 1 μm, and an EC50 of 0.14 μm (Fig. 5). PMA (1 μm) alone elevated phospho-CREB levels near those observed with maximal stimulation by forskolin. In addition, as shown in Fig. 5 (inset and representative blot image), PMA potentiated the effect of subthreshold concentrations of forskolin. The concentration-response curve for PMA alone or in combination with a low concentration of forskolin (0.1 μm) on CREB phosphorylation at Ser 133 is shown in Fig. 6A. PMA caused a dose-dependent increase in phospho-CREB, reaching levels of more than 15-fold the basal values with 1 μm, the highest concentration used. Forskolin alone at 0.1 μm induced minor increases in phospho-CREB levels but significantly potentiated the stimulatory effect of all PMA concentrations used (Fig. 6A).
Figure 5.
Synergistic effect of forskolin (FSK) and PMA on CREB phosphorylation. Cultured 4B cells were incubated for 30 min with increasing concentrations of forskolin in the presence and absence of 1 μm PMA. Data points are the mean and se of the values obtained by Western blot using an antibody against phospho Ser133-CREB in six experiments. The larger-scale representation of the low forskolin concentrations is depicted in the inset. Two-way ANOVA revealed marked increases in Ser133-phospho-CREB with PMA alone and a potentiation of the stimulatory effect of low concentrations of forskolin on CREB phosphorylation. *, P < 0.05 vs. forskolin alone; **, P < 0.01 vs. basal or forskolin alone. TFIIB, Transcription factor IIB.
Figure 6.
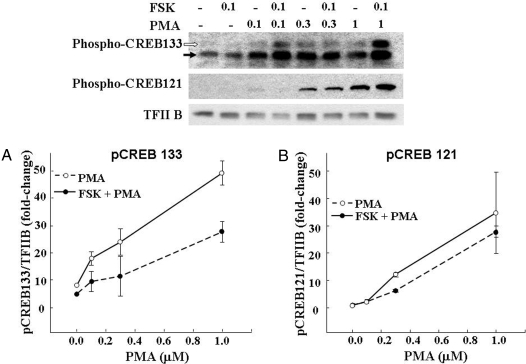
The effect of PMA on CREB phosphorylation (pCREB) at Ser133 (A) and Ser121 (B). 4B cells were incubated for 30 min with increasing concentrations of PMA in the presence and absence of a threshold concentration of forskolin (FSK; 0.1 μm). PMA induced CREB phosphorylation at both sites with a similar concentration dependence. Data points are the mean and se of values obtained by Western blot in two experiments.
To determine whether different phosphorylation patterns of CREB could account for the inability of PMA to stimulate CRH transcription, we measured levels of phospho-Ser 121 CREB in 4B cells exposed to forskolin and/or PMA. As shown in Fig. 6B, PMA increased Ser 121-CREB phosphorylation with a concentration dependence similar to that observed for Ser 133-CREB. However, in contrast to the effects on Ser 133, 0.1 μm forskolin had no effect on basal or 0.1 μm PMA-stimulated Ser 121-CREB phosphorylation and only tended to potentiate the effect of higher concentrations of PMA in the overall analysis by two-way ANOVA. Analysis by t test showed significant potentiation by forskolin only at 0.3 μm PMA.
Effect of signaling inhibitors on phospho-CREB levels and CRH transcription
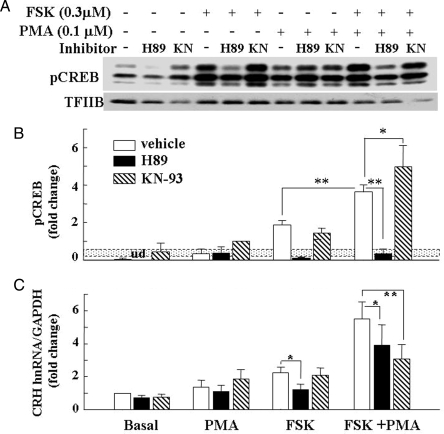
Consistent with experiments above, basal phospho-CREB levels were very low in 4B cells. A low concentration of PMA (0.1 μm) resulted in a small but consistent increase in phospho-CREB. A significant increase in CREB phosphorylation, 5.7-fold higher than that observed with 0.1 μm PMA alone, was noted after incubation of the cells with 0.3 μm forskolin (Fig. 7). Incubation with both stimuli combined resulted in further stimulation (P < 0.01), compared with forskolin alone (Fig. 7A). The PKA inhibitor, H89, abolished basal and forskolin-stimulated CREB phosphorylation and reduced the effect of the combination of PMA and forskolin to levels observed with PMA alone. As expected, H89 had no effect on CREB phosphorylation induced by PMA alone. Interestingly, the calcium-calmodulin-dependent kinase, KN-93, significantly increased the synergistic effect of forskolin and PMA on phospho-CREB levels (P < 0.05, compared with forskolin plus PMA in the presence of vehicle).
Figure 7.
Effect of the PKA inhibitor, H89, and the calcium calmodulin-dependent kinase, KN93, on Ser133 CREB phosphorylation (A and B) and CRH transcription (C). Cells were incubated for 30 min (Western, phospho-CREB detection) or 45 min (real-time PCR, CRH hnRNA detection) in the presence or absence of 0.3 μm forskolin (FSK), 0.1 μm PMA, or their combination 30 min after addition of the inhibitor (1 μm H89 or 10 μm KN-93). The dotted box in B indicates the se of phospho-CREB induced by 0.1 μm PMA alone, the value used to calculate the fold change because in some of the experiments basal phospho-CREB levels were undetectable. The line in C shows the basal CRH hnRNA levels measured by intronic qRT-PCR. Bars are the mean and se of the values obtained in four experiments. *, P < 0.05; **, P < 0.01. TFIIB, Transcription factor IIB.
Initial experiments to study the effect of signaling inhibitors in 4B cells transfected with the CRH promoter-luciferase construct revealed marked nonspecific effects of the PKA inhibitor, H89, and the calcium calmodulin-dependent kinase inhibitor, KN-93, on the activity of the promoterless vector (pGL3 basic). Therefore, the effects of H89 and KN93 on CRH transcription were studied only in primary hypothalamic cells cultures (Fig. 7B). Basal CRH hnRNA levels were unaffected by either the PKA inhibitor, H89, or the calcium calmodulin-dependent kinase, KN-93. As in the experiments above, CRH hnRNA levels were unaffected by 0.1 μm PMA alone, increased by 2.2-fold with 0.3 μm forskolin alone, and increased by 4.4-fold with the combination of the two stimuli. The PKA inhibitor, H89, inhibited forskolin-stimulated CRH hnRNA to near basal levels but had a much smaller inhibitory effect on the stimulated levels by the combination of PMA and forskolin (29% inhibition, P < 0.05). The calcium calmodulin-dependent kinase inhibitor, KN-93, had no effect on forskolin-stimulated CRH hnRNA but inhibited the stimulatory action of forskolin plus PMA by 45% (P < 0.01, compared with values in the absence of inhibitor). The ERK kinase inhibitors, SL327 and UO126, had no significant effect on basal or stimulated CRH hnRNA levels in primary hypothalamic cultures or CRH promoter activity in 4B cells transfected with CRH promoter-driven luciferase construct (data not shown).
Effect of the CREB dominant negative, A-CREB, on CRH transcription
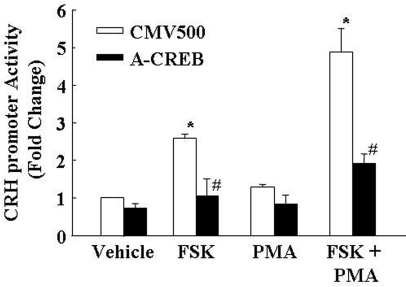
The role of phospho-CREB on the activation of the CRH promoter by forskolin and PMA in 4B cells was examined using the CREB dominant negative, A-CREB (Fig. 8). In cells cotransfected with the empty expression vector (CMV500), forskolin increased CRH promoter activity by 2.6-fold. PMA alone had no significant effect, but when combined with forskolin, it potentiated the effect, increasing CRH promoter activity by 4.9-fold. Cotransfection of the cells with A-CREB prevented the stimulatory effect of forskolin alone on CRH promoter activity and markedly reduced the stimulatory effect of combined PMA and forskolin (from 4.9- to 2.0-fold the basal levels) (Fig. 8).
Figure 8.
Effect of the CREB dominant-negative A-CREB on forskolin with or without PMA on CRH promoter activity. 4B cells cotransfected with the CRH promoter-luciferase construct and A-CREB or the empty vector (CMV500) were incubated with forskolin (FSK), PMA, or their combination for 6 h before measurement of luciferase activity. Bars are the mean and se of the values obtained in four experiments. *, P < 0.01 vs. vehicle CMV control; #, P < 0.01 lower than the respective CMV control.
Discussion
The major findings in these studies are, first, that activation of calcium phospholipid-dependent pathways by PMA potentiates the ability of small elevations of intracellular cAMP to activate CRH transcription, and second, that phosphorylation of CREB is required but not sufficient for activation of CRH transcription. Progress on the elucidation of the mechanisms regulating CRH transcription in the hypothalamus has been slow because of the lack of cell lines representative of parvocellular CRH neurons. The cell line, 4B, used in these studies was originally characterized as expressing low levels of CRH mRNA and CRH-like immunoreactivity, which coelutes with a CRH standard by reverse-phase HPLC analysis (21). However, in current passages of the cells, there is minor or null expression endogenous CRH, making the cells suitable only for reporter gene assays (19). Despite these problems, the present experiments using 4B cells transfected with a CRH promoter-driven luciferase construct provide useful information on the interaction of cAMP-dependent and independent pathways regulating CRH transcription. Previous studies using these cells and AtT-20 cells transfected with a CRH promoter-driven reporter luciferase construct revealed that minor increases in intracellular cAMP are sufficient for activation of CRH transcription (9). Here, we confirmed this observation and also demonstrated that PMA, which is completely ineffective on its own, potentiates the activating effect of forskolin on CRH promoter activity. To confirm the relevance of this interaction in the regulation of CRH transcription in the paraventricular nucleus, we set up a procedure to measure changes of endogenous CRH primary transcript in neuronal cultures. Measurement of changes in CRH hnRNA by in situ hybridization has been extensively used as an accepted index of changes in transcription (22,23,24,25,26). The preset study shows that intronic qRT-PCR is a useful tool to measure hnRNA levels in primary cultures of hypothalamic cells. These experiments confirmed the primary role of cAMP regulating CRH transcription (7,8,19,27,28) and demonstrated that synergism between cAMP-dependent and -independent pathways does occur in hypothalamic CRH neurons. Interestingly, the time relationship of the changes in CRH hnRNA levels after exposure to forskolin was transient, resembling the pattern observed during stress in vivo (29).
An important issue is the mechanism by which PMA potentiates forskolin-stimulated CRH transcription. Previous studies have shown that cAMP is a key regulator of CRH transcription and that the CRE located at −227 is essential for cAMP, dependent and independent of CRH transcription (6,7,9,19,27). Consistent with previous reports in other systems (30,31), in 4B cells and primary hypothalamic neuronal cultures, PMA potentiated the increases of intracellular cAMP accumulation induced by forskolin. This potentiation of cAMP production could contribute to the synergistic action of PMA and forskolin on CRH transcription. However, this is unlikely because in 4B cells, very low cAMP levels are sufficient for full activation of CRH promoter activity (9). Moreover, potentiation of cAMP production by PMA was observed with concentrations of forskolin above threshold for activation of the CRH promoter. According to the conventional models, cAMP activates PKA, leading to sequential phosphorylation of CREB, binding of phospho-CREB to the −227 CRE in the CRH promoter, and activation of transcription (7,8,29). However, in the present experiments, PMA induced CREB phosphorylation and potentiated the effect of forskolin, but it is clear that the increases in phospho-CREB alone are not sufficient to stimulate CRH transcription. Thus, the failure of PMA-induced CREB phosphorylation to induce CRH transcription in 4B cells or primary hypothalamic neuronal cultures, indicate that phospho-CREB must interact with an additional factor to activate the CRH promoter.
In keeping with the PKA dependence of forskolin-induced CREB phosphorylation, the PKA inhibitor, H89, prevented CREB phosphorylation by forskolin and reduced the effect of forskolin plus PMA to the levels observed with PMA alone. Although the blockade of CREB phosphorylation by H89 also prevented the increase in CRH hnRNA by forskolin, the present experiments provide clear evidence that there is no correlation between levels of CREB phosphorylation and activation of CRH transcription. First, the lack of effect of H89 and A-CREB on basal CRH hnRNA, despite suppressing phospho-CREB, suggests that resting levels of CRH expression are independent of phospho-CREB. Second, as discussed earlier, the inability of PMA-stimulated CREB phosphorylation to increase CRH promoter activity or CRH hnRNA indicates that phospho-CREB alone is not sufficient for stimulating transcription. Third, the effects of H89 on cells stimulated with both PMA and forskolin show that minor phospho-CREB induction by a low concentration of PMA (which is H89 resistant) is sufficient for activation of CRH transcription to levels higher than those observed with forskolin alone. Lastly, KN-93 reduced forskolin-stimulated CRH transcription and stimulated CREB phosphorylation. All this evidence suggests that activation of CRH transcription by phospho-CREB requires an additional coactivator and that the presence of such a factor is permissive for transcriptional activation by small elevations in phospho-CREB. Such a requirement for a coactivator has been demonstrated for phospho-CREB-dependent regulation of specific genes (32,33). In this regard, activation of gluconeogenesis by glucagon in the liver involves cAMP/CREB-dependent transcriptional activation of the gluconeogenic enzymes, phosphoenol pyruvate carboxykinase, glucose-6-phosphatase, and peroxisome proliferation-activated receptor-γ coativator-1. However, insulin, which has opposite effects on gluconeogenic enzyme expression, also increases CREB phosphorylation (34). This opposite regulation depends on diverse effects of both regulators to activate the CREB coactivator, transducer of regulated CREB activity (TORC)-2 (34). The coactivator, TORC, is also required for CREB-mediated activation of other cAMP-regulated genes, such as steroidogenic acute regulatory protein, amphiregulin, hepatic leukemia factor, regulator of G protein-coupled receptor 2, and nuclear receptor subfamily 5, group A, member 1 (33,35,36). Whereas future studies should reveal the extent to which TORC is the required coactivator for phospho-CREB stimulation of CRH transcription, it is clear from the data that the coactivator is cAMP but not PKA dependent.
Whereas the evidence above clearly shows that phospho-CREB alone cannot activate CRH transcription, the experiments using the PKA inhibitor, H89, and the CREB dominant negative, A-CREB, indicate that CREB phosphorylation is essential for activation of CRH transcription by cAMP. However, it is not clear from these experiments whether CREB phosphorylation is an absolute requirement for activation of CRH transcription by the combination of PMA and forskolin. The failure of H89 to suppress transcriptional activation by forskolin plus PMA is not surprising, assuming that non-PKA-dependent CREB phosphorylation by PMA is sufficient for CRH promoter transactivation. On the other hand, the CREB dominant negative, A-CREB, should have prevented CREB-mediated transcription irrespective of the pathway leading to CREB phosphorylation. However, in only two of five experiments, A-CREB totally prevented CRH promoter activation by forskolin plus PMA. This could be due to variability in transfection efficiency of A-CREB because gel shift assays, using CRH-CRE oligonucleotides and nuclear extracts from A-CREB-transfected cells treated with forskolin and PMA, revealed residual CREB binding in two of three experiments (data not shown). Because H89 inhibits luciferase activity in the promoterless vector and difficulties in transfecting A-CREB in primary neuronal cultures, we cannot rule out the possibility that the combination of forskolin and PMA could drive transcription in a phospho-CREB-independent manner. However, it is clear from the data that CREB phosphorylation is essential for full transcriptional activation.
In summary, the study shows that activation of calcium phospholipid-dependent pathways by PMA potentiates the ability of small elevations of intracellular cAMP to induce CRH transcription, suggesting that such interaction plays a role in the physiological regulation of CRH expression. In addition, the data demonstrate that phosphorylation of CREB is required but not sufficient for activation of CRH transcription and that full transcriptional activation requires a coactivator, which must interact with phospho-CREB at the CRH promoter to allow transcriptional activation.
Footnotes
This work was supported by the Intramural Research Program, National Institute of Child Health and Human Development, National Institutes of Health.
Disclosure Statement: The authors have nothing to disclose.
First Published Online March 27, 2008
Abbreviations: CRE, cAMP response element; CREB, CRE binding protein; GAPDH, glyceraldehyde 3-phosphate dehydrogenase; hn, heteronuclear; IBMX, isobutyl-methylxanthine; phospho, phosphorylated; PKA, protein kinase A; PMA, phorbol-12-myristate-13-acetate; qRT-PCR, quantitative RT-PCR; TORC, transducer of regulated CREB activity.
References
- Aguilera G 1994 Regulation of pituitary ACTH secretion during chronic stress. Front Neuroendocrinol 15:321–350 [DOI] [PubMed] [Google Scholar]
- Antoni FA 1986 Hypothalamic control of adrenocorticotropin secretion: advances since the discovery of 41-residue corticotropin-releasing factor. Endocr Rev 7:351–378 [DOI] [PubMed] [Google Scholar]
- Sapolsky RM, Romero LM, Munck AU 2000 How do glucocorticoids influence stress responses? Integrating permissive, suppressive, stimulatory, and preparative actions. Endocr Rev 21:55–89 [DOI] [PubMed] [Google Scholar]
- Aguilera G 1998 Corticotropin releasing hormone, receptor regulation and the stress response. Trends Endocrinol Metab 9:329–336 [DOI] [PubMed] [Google Scholar]
- Herbert J, Goodyer IM, Grossman AB, Hastings MH, de Kloet ER, Lightman SL, Lupien SJ, Roozendaal B, Seckl JR 2006 Do corticosteroids damage the brain? J Neuroendocrinol 18:393–411 [DOI] [PubMed] [Google Scholar]
- Guardiola-Diaz HM, Boswell C, Seasholtz AF 1994 The cAMP-responsive element in the corticotropin-releasing hormone gene mediates transcriptional regulation by depolarization. J Biol Chem 269:14784–14791 [PubMed] [Google Scholar]
- Seasholtz AF, Thompson RC, Douglass JO 1988 Identification of a cyclic adenosine monophosphate-responsive element in the rat corticotropin-releasing hormone gene. Mol Endocrinol 2:1311–1319 [DOI] [PubMed] [Google Scholar]
- Wolfl S, Martinez C, Majzoub JA 1999 Inducible binding of cyclic adenosine 3′,5′-monophosphate (cAMP)-responsive element binding protein (CREB) to a cAMP-responsive promoter in vivo. Mol Endocrinol 13:659–669 [DOI] [PubMed] [Google Scholar]
- Nikodemova M, Kasckow J, Liu H, Manganiello V, Aguilera G 2003 Cyclic adenosine 3′,5′-monophosphate regulation of corticotropin-releasing hormone promoter activity in AtT-20 cells and in a transformed hypothalamic cell line. Endocrinology 144:1292–1300 [DOI] [PubMed] [Google Scholar]
- Herman JP, Prewitt CM, Cullinan WE 1996 Neuronal circuit regulation of the hypothalamo-pituitary-adrenocortical stress axis. Crit Rev Neurobiol 10:371–394 [DOI] [PubMed] [Google Scholar]
- Palkovits, Baffi, Pacak 1999 The role of ascending neuronal pathways in stress-induced release of noradrenaline in the hypothalamic paraventricular nucleus of rats. J Neuroendocrinol 11:529–539 [DOI] [PubMed] [Google Scholar]
- Feuvrier E, Aubert M, Mausset AL, Alonso G, Gaillet S, Malaval F, Szafarczyk A 1998 Glucocorticoids provoke a shift from α2- to α1-adrenoreceptor activities in cultured hypothalamic slices leading to opposite noradrenaline effect on corticotropin-releasing hormone release. J Neurochem 70:1199–1209 [DOI] [PubMed] [Google Scholar]
- Feuvrier E, Aubert M, Malaval F, Szafarczyk A, Gaillet S 1999 Opposite regulation by glucocorticoids of the α1B- and α2A-adrenoreceptor mRNA levels in rat cultured anterior hypothalamic slices. Neurosci Lett 271:121–125 [DOI] [PubMed] [Google Scholar]
- Plotsky PM, Cunningham ETJ, Widmaier EP 1989 Catecholaminergic modulation of corticotropin-releasing factor and adrenocorticotropin secretion. Endocr Rev 10:437–458 [DOI] [PubMed] [Google Scholar]
- Costa-Martinez M, Fiber JM, Brown RD, Etgen AM 1999 Localization of α1B-adrenergic receptor in female rat brain regions involved in stress and neuroendocrine function. Neurochem Int 35:383–391 [DOI] [PubMed] [Google Scholar]
- Day H, Campeau S, Watson SJ, Akil H 1999 Expression of α(1b) adrenoceptor mRNA in corticotropin-releasing hormone-containing cells of the rat hypothalamus and its regulation by corticosterone. J Neurosci 19:10098–10106 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pieribone VA, Nicholas AP, Dagerlind A, Hokfelt T 1994 Distribution of α1 adrenoceptors in rat brain revealed by in situ hybridization experiments utilizing subtype-specific probes. J Neurosci 14:4252–4268 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cotecchia S, Kobilka BK, Daniel KW, Nolan RD, Lapetina EY, Caron MG, Lefkowitz RJ, Regan JW 1990 Multiple second messenger pathways of α-adrenergic receptor subtypes expressed in eukaryotic cells. J Biol Chem 265:63–69 [PubMed] [Google Scholar]
- Liu Y, Kalintchenko N, Sassone-Corsi P, Aguilera G 2006 Inhibition of corticotrophin-releasing hormone transcription by inducible cAMP-early repressor in the hypothalamic cell line, 4B. J Neuroendocrinol 18:42–49 [DOI] [PubMed] [Google Scholar]
- Livak KJ, Schmittgen TD 2001 analysis of relative gene expression data using real-time quantitative PCR and the 2-ΔΔCT method. Methods 25:402–408 [DOI] [PubMed] [Google Scholar]
- Kasckow J, Mulchahey JJ, Aguilera G, Pisarska M, Nikodemova M, Chen HC, Herman JP, Murphy EK, Liu Y, Rizvi TA, Dautzenberg FM, Sheriff S 2003 Corticotropin-releasing hormone (CRH) expression and protein kinase A mediated CRH receptor signalling in an immortalized hypothalamic cell line. J Neuroendocrinol 15:521–529 [DOI] [PubMed] [Google Scholar]
- Ma XM, Levy A, Lightman SL 1997 Rapid changes in heteronuclear RNA for corticotrophin-releasing hormone and arginine vasopressin in response to acute stress. J Endocrinol 152:81–89 [DOI] [PubMed] [Google Scholar]
- Ma XM, Aguilera G 1999 Differential regulation of corticotropin-releasing hormone and vasopressin transcription by glucocorticoids. Endocrinology 140:5642–5650 [DOI] [PubMed] [Google Scholar]
- Watts AG, Sanchez-Watts G 2002 Interactions between heterotypic stressors and corticosterone reveal integrative mechanisms for controlling corticotropin-releasing hormone gene expression in the rat paraventricular nucleus. J Neurosci 22:6282–6289 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cole RL, Sawchenko PE 2002 Neurotransmitter regulation of cellular activation and neuropeptide gene expression in the paraventricular nucleus of the hypothalamus. J Neurosci 22:959–969 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herman JP, Schafer MK, Thompson RC, Watson SJ 1992 Rapid regulation of corticotropin-releasing hormone gene transcription in vivo. Mol Endocrinol 6:1061–1069 [DOI] [PubMed] [Google Scholar]
- Guardiola-Diaz HM, Kolinske JS, Gates LH, Seasholtz AF 1996 Negative glucocorticoid regulation of cyclic adenosine 3′, 5′-monophosphate-stimulated corticotropin-releasing hormone-reporter expression in AtT-20 cells. Mol Endocrinol 10:317–329 [DOI] [PubMed] [Google Scholar]
- King BR, Nicholson RC 2007 Advances in understanding corticotrophin-releasing hormone gene expression. Front Biosci 12:581–590 [DOI] [PubMed] [Google Scholar]
- Shepard JD, Liu Y, Sassone-Corsi P, Aguilera G 2005 Role of glucocorticoids and cAMP-mediated repression in limiting corticotropin-releasing hormone transcription during stress. J Neurosci 25:4073–4081 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Abou-Samra AB, Harwood JP, Manganiello VC, Catt KJ, Aguilera G 1987 Phorbol 12-myristate 13-acetate and vasopressin potentiate the effect of corticotropin-releasing factor on cyclic AMP production in rat anterior pituitary cells. Mechanisms of action. J Biol Chem 262:1129–1136 [PubMed] [Google Scholar]
- Vanecek J, Sugden D, Weller JL, Klein DC 1986 See-saw signal processing in pinealocytes involves reciprocal changes in the α1-adrenergic component of the cyclic GMP response and the β-adrenergic component of the cyclic AMP response. J Neurochem 47:678–686 [DOI] [PubMed] [Google Scholar]
- Conkright MD, Canettieri G, Screaton R, Guzman E, Miraglia L, Hogenesch JB, Montminy M 2003 TORCs: transducers of regulated CREB activity. Mol Cell 12:413–423 [DOI] [PubMed] [Google Scholar]
- Xu W, Kasper LH, Lerach S, Jeevan T, Brindle PK 2007 Individual CREB-target genes dictate usage of distinct cAMP-responsive coactivation mechanisms. EMBO J 26:2890–2903 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dentin R, Liu Y, Koo SH, Hedrick S, Vargas T, Heredia J, Yates J, Montminy M 2007 Insulin modulates gluconeogenesis by inhibition of the coactivator TORC2. Nature 449:366–369 [DOI] [PubMed] [Google Scholar]
- Takemori H, Kanematsu M, Kajimura J, Hatano O, Katoh Y, Lin XZ, Min L, Yamazaki T, Doi J, Okamoto M 2007 Dephosphorylation of TORC initiates expression of the StAR gene. Mol Cell Endocrinol 265–266:196–204 [DOI] [PubMed] [Google Scholar]
- Siu YT, Chin KT, Siu KL, Yee Wai Choy E, Jeang KT, Jin DY 2006 TORC1 and TORC2 coactivators are required for tax activation of the human T-cell leukemia virus type 1 long terminal repeats. J Virol 80:7052–7059 [DOI] [PMC free article] [PubMed] [Google Scholar]