Abstract
Many painful conditions occur more frequently in women, and estrogen is a predisposing factor. Estrogen may contribute to some pain syndromes by enhancing axon outgrowth by sensory dorsal root ganglion (DRG) neurons. The objective of the present study was to define mechanisms by which estrogen elicits axon sprouting. The estrogen receptor-α agonist propyl pyrazole triol induced neurite outgrowth from cultured neonatal DRG neurons, whereas the estrogen receptor-β agonist diarylpropionitrile was ineffective. 17β-Estradiol (E2) elicited sprouting from peripherin-positive unmyelinated neurons, but not larger NF200-positive myelinated neurons. Microarray analysis showed that E2 up-regulates angiotensin II (ANGII) receptor type 2 (AT2) mRNA in vitro, and studies in adult rats confirmed increased DRG mRNA and protein in vivo. AT2 plays a central role in E2-induced axon sprouting because AT2 blockade by PD123,319 eliminated estrogen-mediated sprouting in vitro. We assessed whether AT2 may be responding to locally synthesized ANGII. DRG from adult rats expressed mRNA for renin, angiotensinogen, and angiotensin converting enzyme (ACE), and protein products were present and occasionally colocalized within neurons and other DRG cells. We determined if locally synthesized ANGII plays a role in estrogen-mediated sprouting by blocking its formation using the ACE inhibitor enalapril. ACE inhibition prevented estrogen-induced neuritogenesis. These findings support the hypothesis that estrogen promotes DRG nociceptor axon sprouting by up-regulating the AT2 receptor, and that locally synthesized ANGII can induce axon formation. Therefore, estrogen may contribute to some pain syndromes by enhancing the pro-neuritogenic effects of AT2 activation by ANGII.
MANY PAIN SYNDROMES are far more prevalent in premenopausal females than in men or postmenopausal women (1,2). For example, the likelihood of women suffering from painful conditions is 3-fold for migraines, 4-fold for temporomandibular pain, 6-fold for fibromyalgia, and 10-fold for interstitial cystitis. The increased occurrence of at least some painful conditions has been linked to higher levels of estrogen [estradiol (E2)] (3). Elevated E2, either through endogenous cycling or as a result of administration of exogenous compounds, is correlated with increased incidence and symptom severity in temporomandibular disorder, orofacial pain, fibromyalgia, rheumatoid arthritis, low back pain, and irritable bowel syndrome (4,5). Thus, emerging evidence supports the idea that E2 may predispose individuals to certain female-associated pain syndromes.
Although multiple factors are likely to be involved in the establishment of chronic pain, accumulating evidence suggests that peripheral axon remodeling may play an important role. Histological studies show that chronically painful conditions such as in lower back pain, tendonitis, and cancer pain are associated with increased numbers of axons (6,7,8), particularly presumptive heat and pain-sensing nociceptor C fibers containing the neuropeptide calcitonin gene-related peptide (CGRP). Therefore, altered nociceptor receptive field properties due to increased fiber branching may contribute to increased pain under some conditions.
The extent to which sensory nerve proliferation contributes to E2-associated pain has not been established. However, nociceptor hyperinnervation is implicated in a number of female pain syndromes, including breast pain, irritable bowel syndrome, interstitial cystitis, and vulvodynia (9,10,11). Although no causal link has been established, E2 is known to promote axon regeneration and sprouting in a number of neural tissues (12,13), including the dorsal root ganglion (DRG) (14,15). Accordingly, E2 could contribute to the onset or severity of some female-related pain syndromes by promoting hyperinnervation of peripheral tissues.
In the present study, we characterized mechanisms by which E2 promotes sprouting of DRG presumptive nociceptor neurons. These studies show that E2 increases expression of the angiotensin II receptor type 2 (AT2) in DRG neurons, and that activation of this receptor is essential for E2 to induce axonal sprouting. Furthermore, our findings demonstrate the presence of an intrinsic DRG renin-angiotensin system (RAS), and show that synthesis of angiotensin II (ANGII) is necessary for E2 to induce sensory neuron outgrowth.
Materials and Methods
All animal protocols and procedures were approved by the Kansas University Medical Center’s Animal Care and Use Committee, and were in accordance with the National Institutes of Health guidelines for the care and use of laboratory animals.
DRG neuronal cultures
Culture experiments were conducted on T8-L4 DRG neurons obtained bilaterally from Sprague Dawley rat pups less than 24 h after birth (Harlan Teklad, Madison, WI). Previous studies have shown that these neonatal neurons express estrogen receptors (ERs) similar to that of adults (16), provide superior yield and purity, and represent a highly responsive system in which to assess the effects of E2 on neuritogenesis (14). Cultures included both female and male pups because no differences were detected in basal or E2-stimulated neurite outgrowth (data not shown). Neurons were dissociated and suspended at a concentration of 10 ganglia per ml in defined Neurobasal A media (Invitrogen Corp., Carlsbad, CA) (14). Neurons (∼15,000 per well) were plated onto laminin-coated 48-well plates (BD Biosciences, San Jose, CA) and maintained at 37 C in 5% CO2 in Neurobasal A media containing 1 nm 17β-E2 (Sigma-Aldrich, St. Louis, MO) or 10 μl/ml ethanol vehicle. Reagents were from Invitrogen unless otherwise indicated.
Microarray analysis of gene expression in cultured DRG neurons
Neurons cultured for 24 h were lysed with 125 μl Trizol per well, lysates from four wells were pooled, and RNA was extracted using Phase Lock Gel Heavy tubes (Eppendorf AG, Hamburg, Germany). Total RNA was prepared (Invitrogen) and evaluated with an Agilent 2100 Bioanalyzer (Agilent Technologies, Inc., Palo Alto, CA). cRNA target was prepared using the Affymetrix Small Sampling Labeling Protocol vII (Affymetrix, Inc., Santa Clara, CA) (http://www2.kumc.edu/siddrc/microarray/protocols.html). cRNA from four pooled E2-treated and four control cultures was hybridized to Affymetrix rat 230 v2 high-density GeneChips and scanned with a GeneChip 3000 High-Resolution Scanner (Affymetrix); these arrays contain 25-mer probe sets interrogating more than 30,000 transcripts. Data were analyzed using Affymetrix MAS5 and GeneSpring (Silicon Genetics, San Carlos, CA) software. Data from microarray experiments were normalized per gene and chip. Genes present in E2-treated samples (i.e. detection P value ≤ 0.05) were ranked according to fold change. Signal intensities for probe sets in the four control and four treatment chips were compared statistically [Linear Models for Microarray Data (17)] with differences considered significant at P ≤ 0.05.
In vivo analysis of DRG gene expression
Female Sprague Dawley rats at approximately 60 d were anesthetized (ip injection of 60 mg/kg ketamine HCl, Ketaject, 0.4 mg/kg atropine sulfate, and 8 mg/kg xylazine Xyla-Ject) and bilaterally ovariectomized (OVX) under aseptic conditions (18). For RNA analysis, OVX rats were injected with either E2-benzoate (10 μg/kg, n = 16) or sesame oil vehicle (n = 16). T8-L4 DRGs were harvested at 24 h postinjection in RNAlater (Ambion, Inc., Austin, TX) and total RNA isolated (NucleoSpin; Macherey-Nagel, Düren, Germany). One microgram of total RNA was reverse transcribed (iScript; Bio-Rad Laboratories, Inc., Hercules, CA). A quantity of 250 ng of reverse-transcribed product were amplified with primers for AT2 (forward 5′-GGCAAGCATCTTATGTAGTTCC-3′; reverse 5′-GAAGGCACTATCACT GAAAGC-3′), angiotensin converting enzyme (ACE) (forward 5′-AACATCACGGAGGAGAATGC-3′; reverse 5′- GCAGATGAGCGG GAATAGG-3′), renin (REN) (forward 5′-CTCTATGACTCCTCGGAATCC-3′; reverse 5′-CCTTGGAGAGCCAGTATGC-3′), angiotensinogen (AGN) (forward 5′-AATAAGGCTGCTTGGTTCAC-3′; reverse 5′-ACTGAGGTGCTGTTGTCC-3′), and glyceraldehyde phosphate dehydrogenase (GAPDH) (forward 5′-CTCTACCCACGG CAAGTTC-3′; reverse 5′-CTCAGCACCAGCATCACC-3′), respectively in 25 μl reaction mixture containing 1× reaction buffer, MgCl2, 0.2 mm deoxynucleotide triphosphates (Invitrogen), 0.3 μm each primer (Integrated DNA Technologies, Coralville, IA), and Platinum Taq DNA Polymerase (Invitrogen) using a Bio-Rad Laboratories iCycler iQ. Primers were designed using Beacon Designer 5.0 (PREMIER Biosoft Intl., Palo Alto, CA). Amplicon lengths and PCR conditions were as follows: AT2 805 bp, MgCl2 1.5 mm, cycle no. 30; ACE 625 bp, MgCl2 1.0 mm, cycle no. 30; REN 713 bp, MgCl2 1.5 mm, cycle no. 30; AGN 983 bp, MgCl2 1.0 mm, cycle no. 32; and GAPDH 130 bp, MgCl2 1.5 mm, cycle no. 30.
All amplification reactions were performed in the linear range, and PCR analyses were conducted in quadruplicate. Relative levels of gene expression were assessed in individual gels by densitometric analysis of product bands (Chemi-Doc; Bio-Rad Laboratories) and normalized by dividing by GAPDH signal in the same lane.
Immunostaining of DRG sections
OVX rats received E2-benzoate (n = 16) or vehicle (n = 16) sc. Twenty-four hours later, rats were deeply anesthetized (100 mg/kg, Nembutal sodium ip; OVATION Pharmaceuticals, Inc., Deerfield, IL), and T8-L4 DRGs were harvested, snap frozen, and cryosectioned at 8 μm. Sections were fixed for 5 min with fresh 4% paraformaldehyde, blocked with donkey or goat serum, and incubated for 18 h at room temperature with goat polyclonal antisera directed against the human AT2 N terminus, which recognizes rat AT2 protein (1:100; Santa Cruz Biotechnology, Inc., Santa Cruz, CA), mouse monoclonal antibody to human ACE with rat protein cross-reactivity (1:600; CHEMICON International, Inc., Temecula, CA), mouse monoclonal antibody to rat AGN (1:1000; Swant, Bellinzona, Switzerland), or rabbit antirat REN antisera (1:3000; a gift from Dr. T. Inagami, Vanderbilt University, Nashville, TN), followed for 1 h by cy3-conjugated antibody (donkey antigoat for AT2, 1:200; goat antirabbit for REN, 1:400; and goat antimouse, 1:200 for ACE and AGN, respectively; Jackson ImmunoResearch Laboratories, Inc., West Grove PA). Some sections were incubated overnight with both anti-AGN (1:800) and anti-REN (1:4000), followed by cy3-conjugated goat antimouse, 1:200 for AGN and cy2-conjugated goat antirabbit, 1:300 for REN. Negative controls included primary antisera preabsorption overnight to a 5-fold excess of blocking peptides for AT2 (Santa Cruz Biotechnology) or REN native protein (BioChain Institute, Inc., Hayward, CA), and primary antisera heat inactivation for 20 min and antibody omission for ACE or AGN. A total of about 12–15 sections spaced throughout each ganglion was inspected using a Nikon Eclipse TE300 inverted microscope (Nikon Corp., Tokyo, Japan) equipped with 10× Plan Fluor 0.30 and 20× Plan Fluor 0.45 objectives, and digital images obtained with an Optronix (Goleta, CA) Magnafire camera and software. In randomly selected sections from two to four ganglia from each of the four OVX or E2-treated rats, neurons were counted to determine the frequency of occurrence of cells immunoreactive for RAS proteins. All neurons displaying a nuclear profile in a given section were counted (96 ± 9 per animal), together with the total number of cells positive for a given antigen. Immunoreactive cells were further characterized on the basis of diameter as small (<20 μm), medium (20–40 μm), or large (>40 μm) and expressed as the percentage of total neurons. Images of cells with double immunofluorescence were captured using a Nikon C1si confocal system equipped with 543 nm HeNe and 488 nm Ar lasers [Melles Griot (Carlsbad, CA) Argon Ion Laser System model 35-IMA 410-015] integrated into an Eclipse 90i microscope with a D-Eclipse camera and 20× Plan Apo 0.75 DIC M/N2 objective. Image acquisition and volume rendering of Z stacks were performed using Nikon EZ-C1–3.4 software.
Pharmacological manipulation of E2-induced neuritogenesis
DRG neurons were cultured in media containing: 1) 1 nm E2 in the presence or absence of the selective ER antagonist, ICI 182,780 (ICI) (10 μm; Tocris, Bristol, UK); 2) the selective ER agonists propyl pyrazole triol (PPT) (10 nm; Tocris) and diarylpropionitrile (DPN) (0.1 nm; Tocris); 3) ANGII (100 nm; Sigma-Aldrich), E2 (1 nm), or nerve growth factor (NGF) (25 ng/ml; Alomone Labs, Ltd., Jerusalem, Israel) in the presence or absence of the selective AT2 blocker PD123,319 (PD) (1.0 μm; Sigma-Aldrich); and 4) the ACE inhibitor enalapril maleate (EM) (10 μm; Sigma-Aldrich) in the presence or absence of E2 (1 nm). Dose-response curves were constructed for ICI, PPT, DPN, and EM to ensure appropriate concentrations. Control cultures received comparable volumes of ethanol vehicle, and all experiments were repeated four or more times.
Immunostaining and analysis of neurite outgrowth in vitro
After 3 d in culture, neurons were fixed with 4% paraformaldehyde (Fisher Scientific, Pittsburgh, PA) for 1 h at room temperature, rinsed with PBS containing 0.3% Triton X-100 (Sigma-Aldrich), and blocked with donkey or goat serum. Cultures were incubated overnight at room temperature with a mouse monoclonal antibody for NF200 (1:200; Sigma-Aldrich) or a rabbit anti-peripherin polyclonal antibody (1:400; CHEMICON) and visualized with cy2-conjugated donkey antimouse (1:200; Jackson ImmunoResearch Laboratories) or cy3-conjugated goat antirabbit IgG antibody (1:400; Jackson ImmunoResearch Laboratories).
For each well, four to seven fields were selected randomly and digital images captured with the inverted microscope. Neurite area was measured using methods essentially identical to those used previously (14). In brief, a stereological grid (AnalySis version 3.2; Soft Imaging System GmBH, Müenster, Germany) was superimposed over each image, and the number of line intersections overlying stained neurites was divided by total intersections within the field and multiplied by total field area. This was divided by the number of neurons with at least one neurite in the field, averaged for each well and expressed as neurite area (μm2) per neuron. A minimum of 1200 neurons was analyzed in each set of culture experiments. Confocal images of the cultured neurons were captured with a Nikon C1 confocal system with Melles Griot Argon Ion Laser model 35-IMA 410-015, 543 nm HeNe and 488 nm Ar lasers, with an Eclipse TE 2000-U microscope, Nikon CoolSNAP K4 camera, and 10× Plan Fluor 0.30 objective. Image acquisition and volume renderings of Z stacks were performed using Nikon EZ-C1-3.4 software.
Statistical analysis
All values are presented as mean ± sem. Statistical comparisons were made using the Student’s t test or one-way ANOVA for normally distributed data, and the Mann-Whitney rank sum test for nonparametric analysis. Post hoc comparisons were made using the Student-Newman-Keuls test. Differences were considered significant at P ≤ 0.05.
Results
E2 promotes sprouting in small but not large DRG neurons
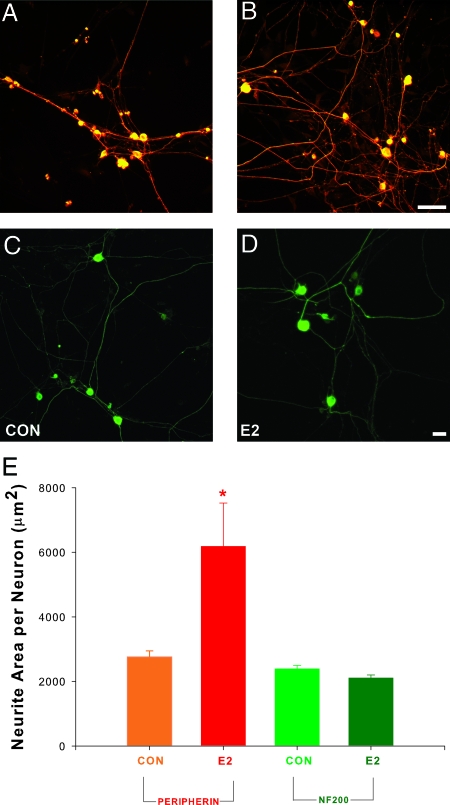
We have shown previously that E2 added to the culture medium increases sprouting by DRG neurons immunoreactive for peripherin and CGRP (14), which comprise predominantly small unmyelinated neurons. It is not clear whether E2 also promotes neurite sprouting by larger myelinated neurons. DRG neurons were cultured for 72 h in serum-free defined media with or without E2. Some cultures were stained for neurofilament 200 (NF200), a marker of myelinated neurons (19), whereas others were stained for peripherin, a cytoskeletal protein marker for small diameter, unmyelinated C-fiber neurons and their neurites (20). E2 enhanced neurite outgrowth in peripherin-immunoreactive (-ir) neurons (Fig. 1, A, B, and E) as reported previously (14). In contrast, neuritogenesis by NF200-ir neurons was comparable in the presence and absence of E2 (Fig. 1, C–E). Therefore, E2 acts selectively to induce neuritogenesis of small unmyelinated DRG neurons.
Figure 1.
E2 selectively promotes axon outgrowth by unmyelinated neurons. Dissociated DRG neurons from rat pups were cultured for 72 h with or without E2. A, Immunostaining for peripherin, a marker of unmyelinated neurons, revealed many neurons and neurites under control culture conditions (CON). B, Outgrowth of peripherin-ir neurites was increased by the addition of 1 nm E2 to the medium. Scale bar, 50 μm for A and B. C, Immunostaining for the myelinated neuronal marker NF200 revealed large neurons with neurites. D, E2 did not appear to increase neurite formation by NF200-ir neurons. Bar, 100 μm for panels C and D. E, Quantitative analysis revealed that E2 significantly increased peripherin-ir neurite area relative to controls (*, P = 0.04) but did not affect neurite formation by NF200-ir neurons. Values are mean ± sem for four cultures in each treatment group.
ERs and neuritogenesis
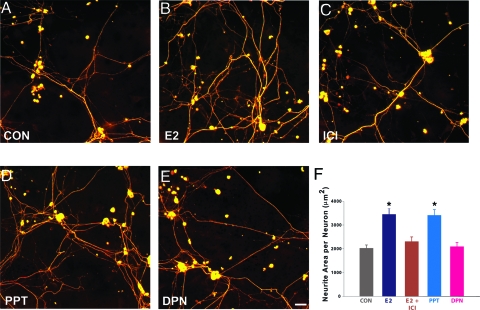
To determine whether classical ERs mediate E2 neuritogenesis, the ER antagonist ICI was added to DRG cultures with or without E2. E2 increased peripherin-ir axon outgrowth by about 70% (Fig. 2, A, B, and F), and this was blocked by ICI (Fig. 2, C and F); ICI alone did not alter basal neurite outgrowth (data not shown).
Figure 2.
E2-induced neuritogenesis by peripherin-immunostained DRG neurons is mediated by the ERα. A, Neurons cultured under control conditions (CON) showed moderate outgrowth after 72 h. B, E2 increased neurite outgrowth. C, Addition of the ER antagonist ICI (10 μm) attenuated E2-induced outgrowth. D, The ERα selective agonist PPT (10 nm) also increased neurite outgrowth. E, Neurite outgrowth did not appear to be affected by the ERβ agonist DPN (0.1 nm). Scale bar in E, 20 μm for A–E. F, Quantitative analysis shows that E2 significantly increases neurite outgrowth and that ICI reduces outgrowth to control levels. Outgrowth induced by PPT is comparable to that of E2, whereas outgrowth in the presence of DPN is comparable to control. Values are means ± sem for four cultures in each treatment group. *, P < 0.02 compared with control conditions, E2 plus ICI, and DPN.
To investigate further which ER mediates neuritogenesis, the ERα-selective agonist PPT or ERβ-selective agonist DPN was added to the media. Neuritogenesis in PPT-treated cultures was significantly greater than controls and comparable to that of E2 (Fig. 2, D and F). In DPN-treated cultures, neuritogenesis was comparable to controls and significantly less than that of E2 or PPT (Fig. 2, E and F). These experiments support the idea that ERα mediates neuritogenesis of cultured DRG neurons.
Estrogen increases AT2 expression in DRG neurons
The preceding findings indicate that E2 increases sprouting selectively by unmyelinated DRG neurons via ERα, which is known to act as a transcription factor (21). To identify candidate genes that may mediate E2 induction of sensory neurite outgrowth, we cultured dissociated DRG neurons in the presence or absence of 1 nm E2 for 24 h. cRNA from pooled samples was hybridized to Affymetrix GeneChips (rat genome 230A v2). Comparable numbers of neurons treated with E2 yielded significantly more total RNA than did neurons receiving vehicle only (306 ± 31 ng/μl vs. 220 ± 9 ng/μl total RNA; P = 0.039). In GeneChips hybridized with the equivalent of 50 ng total RNA, E2-treated samples displayed 141 genes that were present and up-regulated 1.5 fold or more, and 59 genes present and down-regulated 1.5 or more. Among the up-regulated genes, AT2 was increased by 2.24-fold (P = 0.011). Because AT2 promotes regeneration of central and peripheral axons (22,23,24) and induces neurite outgrowth in cultured cells (23,25,26,27), this gene was selected for further investigation.
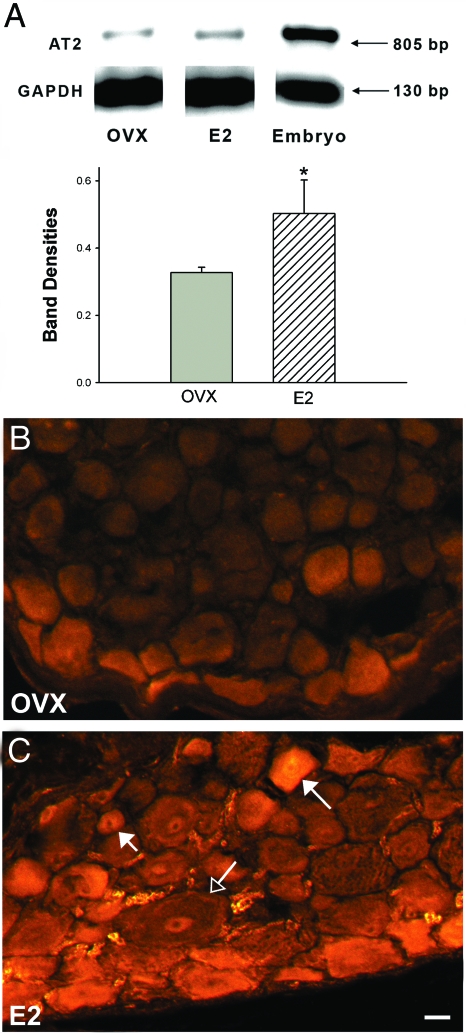
To confirm AT2 gene up-regulation in vivo, 8-wk-old OVX female rats received a single sc injection of E2-benzoate (10 μg/kg; n = 3) or sesame oil vehicle (n = 4). DRGs were harvested after 24 h, and RNA was analyzed by RT-PCR. An 805-bp AT2 amplicon was detected in samples from adult rat DRGs as well as rat embryo positive controls. AT2 mRNA expression was increased by 55% after E2 treatment compared with vehicle (P = 0.02; Fig. 3A).
Figure 3.
17β-E2 up-regulates DRG AT2 mRNA and protein. Adult OVX female rats were treated with E2-benzoate or sesame oil vehicle, and DRG analyzed after 24 h. A, RT-PCR shows AT2 and GAPDH amplicons in OVX and E2-treated rats and rat embryo positive control. Bar graph shows densitometric analysis of the AT2 bands normalized to GAPDH from a single gel. n = 4 for control and n = 3 for treated groups (*, P = 0.02). B, DRG sections from OVX rats show weak AT2 immunostaining mainly in small and medium-sized neurons. C, After E2 treatment, AT2-ir was increased in small (short-tailed arrow) and medium (long-tailed arrow) neurons, but not in large neurons (open arrow). Scale bar, 20 μm.
We assessed AT2 distribution in DRG cells of adult OVX rats 24 h after E2 or vehicle injections by immunostaining for the N-terminus region of AT2. In DRGs of OVX rats (n = 4), AT2-ir was observed in some satellite cells and a small number of small to medium-sized neurons (Fig. 3B). After E2 injection (n = 4), AT2-ir increased substantially in small and medium-sized DRG neurons (Fig. 3C).
AT2 activation is required for E2-induced sensory neuritogenesis
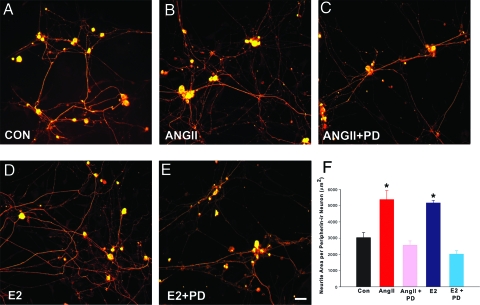
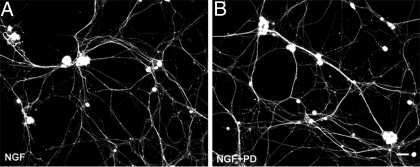
To determine AT2’s role in DRG neuronal sprouting, we first assessed whether angiotensin receptor activation by the native ligand elicits neurite outgrowth. DRG neurons cultured for 72 h in the presence of 100 nm ANGII showed a 46% increase in peripherin-ir axons (Fig. 4, A, B, and F), supporting a role of ANGII receptors in sensory neuritogenesis. To determine if AT2 mediates ANGII-induced neurite outgrowth, DRG neurons were cultured with ANGII and the selective AT2 blocker PD. PD abolished neuritogenesis elicited by ANGII (Fig. 4, C and F), confirming that AT2 activation is required for ANGII-induced sensory neurite outgrowth.
Figure 4.
AT2 mediates estrogen-induced neurite outgrowth by peripherin-immunostained DRG neurons. A, Neurons cultured under control conditions show basal neurite outgrowth. B, Addition of 100 nm ANGII increases neurite outgrowth. C, The selective AT2 blocker PD (1 μm) reduced neurite outgrowth. D, E2 (1 nm) increased neurite outgrowth. E, Addition of 1 μm PD to E2-containing cultures reduced neurite outgrowth. Scale bar, 20 μm in A–E. F, Quantitation confirms that ANGII increases neurite outgrowth, which is reduced by PD, and that E2-induced neuritogenesis is reversed by AT2 inhibition. Values are ± sem (n = 6 culture experiments). *, P < 0.01 for ANGII compared with control conditions (CON) and ANGII plus PD or E2 compared with control conditions and E2 plus PD.
We next assessed whether AT2 activation is required for E2-induced neuritogenesis. Neurons were cultured with E2 in the presence or absence of PD. E2 increased peripherin-ir neurite outgrowth by 86% (Fig. 4, A, D, and F), and PD prevented this increase (Fig. 4, E and F). Neurite area for PD alone (2717 ± 441 μm2) was comparable to the control. Therefore, AT2 activation is required for E2-induced but not basal sensory neuritogenesis.
To determine whether AT2 is involved in neuritogenesis evoked by stimuli other than E2 and ANGII, neurons were cultured in the presence of NGF with or without PD. NGF-induced neurite outgrowth was comparable in the absence (Fig. 5A; 5876 ± 475 μm2) or presence of PD (Fig. 5B; 6244 ± 530 μm2, respectively). Therefore, AT2 activation is not required for NGF-mediated sensory neuritogenesis.
Figure 5.
NGF-induced neurite outgrowth by peripherin-immunostained DRG neurons is not affected by AT2 blockade. A, Neurons cultured in the presence of 25 ng/ml NGF show extensive neurite outgrowth. B, Addition of 1 μm PD to NGF-containing cultures had no effect on the neurite outgrowth induced by NGF alone. Quantitative data are presented in the text. Scale bar, 50 μm.
An intrinsic DRG REN angiotensin system
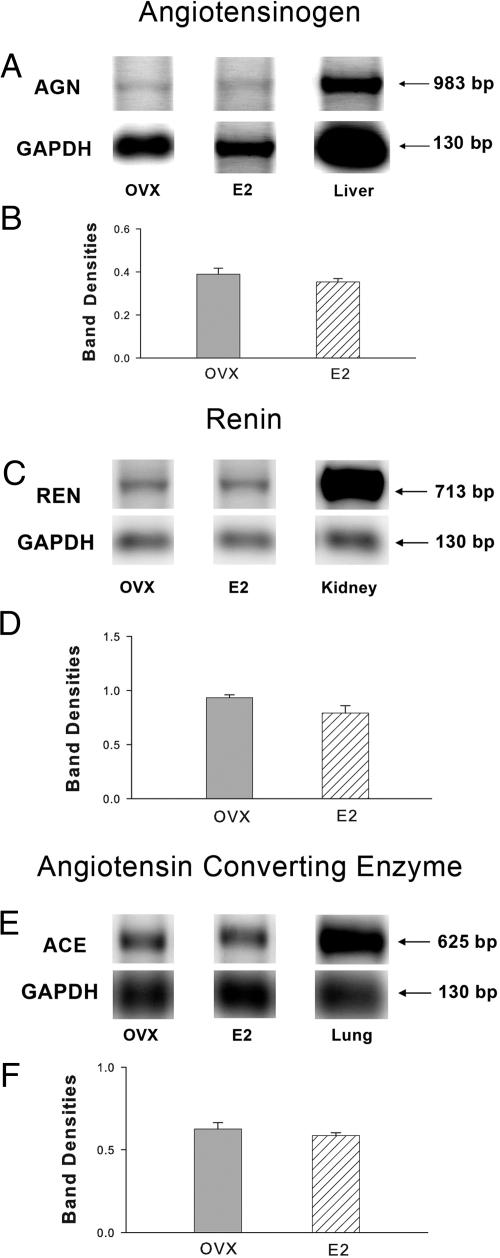
The finding that E2 promotes AT2-mediated DRG neurite outgrowth in serum-free defined cultures raises questions as to the source of the ANGII activating this receptor. To determine if DRG cells contain E2-regulated genes necessary for ANGII synthesis, adult female OVX rats were injected with E2 (n = 4) or vehicle (n = 4), and DRG was analyzed for AGN, REN, and ACE mRNA. RT-PCR showed that AGN, REN, and ACE genes are transcribed in DRGs in vivo (Fig. 6, A, C, and E). When normalized to GAPDH, amplicon levels did not differ between OVX and E2-treated rats (Fig. 6, B, D, and F), indicating that E2 treatment did not affect overall RAS DRG transcription.
Figure 6.
DRGs contain genes encoding an intrinsic RAS. Representative gels showing RT-PCR products, together with densitometric analyses, are presented for AGN (A and B), REN (C and D), and ACE (E and F). RNA was obtained from DRGs of adult rats that were OVX and treated with E2. For quantitative analysis, amplicon bands were normalized to GAPDH, and values represent mean ± sem for four animals per treatment group. Positive controls included the liver for AGN, the kidney for REN, and the lung for ACE.
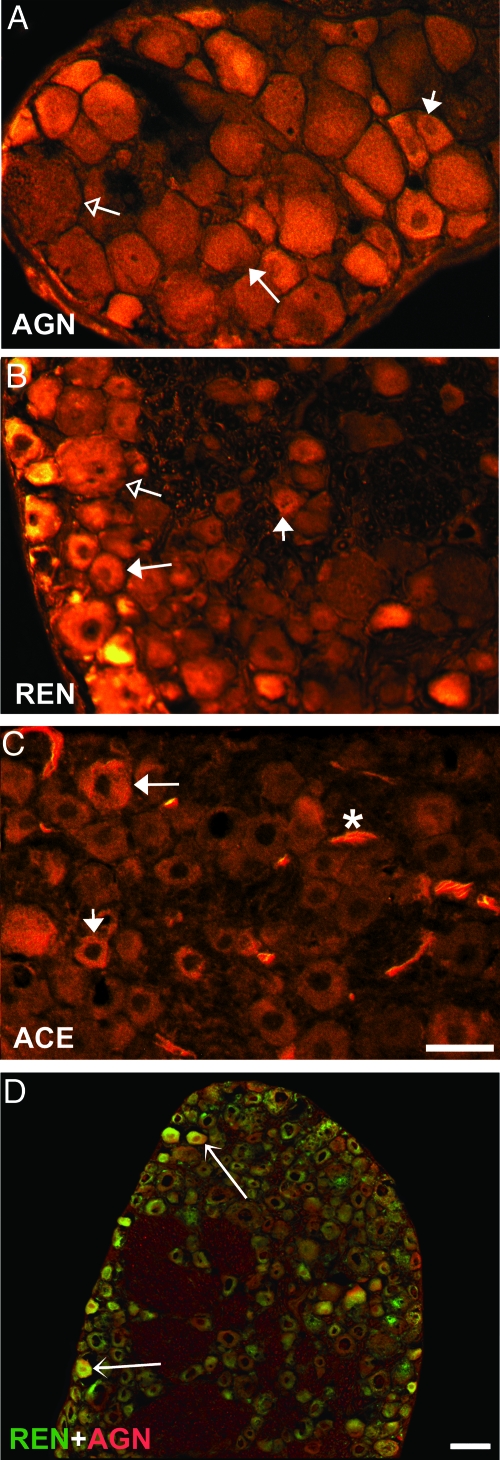
The presence and cellular distribution of RAS protein were assessed in DRG sections incubated with antibodies to AGN, REN, and ACE. In OVX rats, immunostaining for AGN, the precursor to ANGII, showed fluorescence in small and medium-sized neurons and some blood vessels (Fig. 7A and Table 1). Most small, medium, and some large neurons also stained for REN (Fig. 7B), which cleaves angiotensin I (ANGI) from AGN. ACE, which converts ANGI to ANGII, was observed predominantly in medium and small neurons, in many blood vessels, and some satellite cells (Fig. 7C and Table 1). To determine whether RAS proteins coexist within single neurons, sections were double stained for AGN and REN. These proteins colocalized within the cytoplasm of many small to medium-sized neurons (Fig. 7D). E2 treatment did not affect ACE immunoreactivity but caused a modest decrease in the total number of REN-ir neurons, and a shift in AGN-ir from smaller to larger cells (Table 1). These findings reveal that RAS components are present within DRG, appearing in small and medium-sized DRG neurons as well as other cell types.
Figure 7.
RAS proteins are present in neurons from DRG sections in adult female OVX rats. A, Immunostaining for AGN shows cytoplasmic localization within small (short-tailed arrow) and medium (long-tailed arrow) neurons. Large neurons (open arrow) showed little to no AGN immunoreactivity. B, REN-ir is present in most small (short-tailed arrow) and medium (long-tailed arrow), and in some large neurons (open arrow). C, ACE-ir is present in medium-sized (long-tailed arrow) and small neurons (short-tailed arrow). ACE-ir was generally absent in large neurons but was strong in blood vessels (asterisk). Scale bar, 50 μm in A–C. D, Double staining of DRG sections for REN (green) and AGN (red) shows colocalization in small and medium-sized neurons (arrows), whereas others express REN or AGN alone. Scale bar, 50 μm in D.
Table 1.
Distribution of immunoreactivity for AGN, REN, and ACE within DRG neurons
| Immunoreactive neurons (%) | AGN
|
REN
|
ACE
|
|||
|---|---|---|---|---|---|---|
| OVX | E2 | OVX | E2 | OVX | E2 | |
| Total | 59 ± 2 | 62 ± 1 | 92 ± 1 | 80 ± 3a | 44 ± 1 | 46 ± 1 |
| Large | 0 | 7 ± 0a | 7 ± 1 | 10 ± 2 | 1 ± 0 | 0 |
| Medium | 21 ± 2 | 27 ± 1a | 51 ± 2 | 34 ± 4 | 23 ± 3 | 23 ± 2 |
| Small | 38 ± 1 | 28 ± 0a | 34 ± 1 | 36 ± 1 | 20 ± 2 | 23 ± 2 |
Values represent percentages of neurons with nuclear profiles showing immunofluorescence relative to all neurons with nuclear profiles in counts from sections through two to four ganglia in OVX (n = 4) and estrogen-treated (E2, n = 4) rats. Neurons were categorized according to maximum diameter as large (>40 μm), medium (20–40 μm), and small (>20 μm).
P < 0.05 relative to OVX.
ACE inhibition reduces E2-induced neurite outgrowth
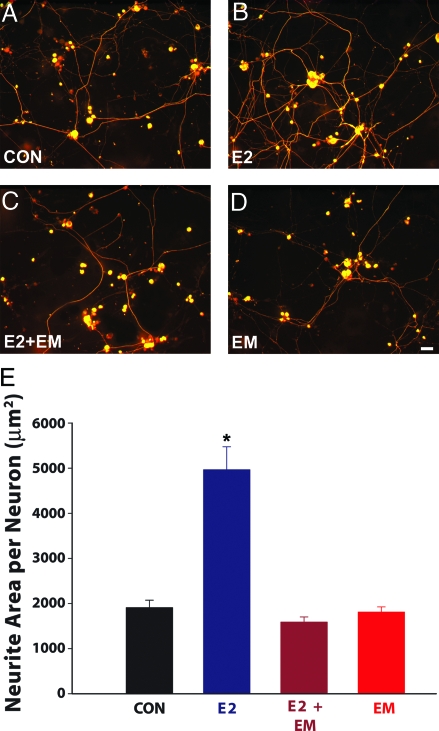
Because all RAS components are present within DRG, locally synthesized ANGII may provide a ligand that, when the AT2 receptor is up-regulated by E2, promotes neurite sprouting. If so, preventing ANGII formation by blocking ACE should inhibit E2-induced neurite outgrowth. Neonatal rat DRG neurons were cultured with or without E2 and in the presence or absence of the ACE inhibitor EM. After 72 h in culture, E2 had increased outgrowth of peripherin-ir neurites significantly (Fig. 8, A, B, and E). EM completely prevented E2-induced outgrowth (Fig. 8, C and E) but did not affect basal outgrowth in the absence of E2 (Fig. 8, D and E). Together, these data indicate that formation of ANGII by ACE is required for E2 to stimulate sensory DRG neurons to increase neurite outgrowth.
Figure 8.
ACE inhibition prevents E2-induced DRG neuritogenesis. A, Cultures lacking amendments (CON) showed basal levels of neurite outgrowth. B, Addition of 1 nm E2 to the culture increased neurite outgrowth. C, The increased neurite outgrowth elicited by E2 was attenuated by adding 10 μm EM to the media. D, Addition of EM alone did not affect neurite outgrowth relative to basal levels. Scale bar, 20 μm for A–D. E, Quantitative analysis of neurite outgrowth showed that E2 significantly increases neurite area, and this is prevented by ACE inhibition. Values are mean ± sem for four cultures per treatment group. *, P < 0.001 compared with cultures lacking amendments, E2 plus EM, and EM, respectively.
Discussion
DRGs contain mixed neuronal populations defined by their differential responsiveness to mechanoreceptive, proprioceptive and nociceptive stimuli. Neurons can also be distinguished by their size and neurochemical properties. Large neurons with Aα/β-myelinated axons and strong NF200 expression are typically associated with mechanoreceptive properties; medium-sized neurons with Aδ-fibers include proprioceptors, and small neurons elaborating C fibers and enriched with peripherin include most nociceptor neurons. Previous culture studies show that E2 promotes sprouting by peripherin-positive DRG neurons (14). This population includes heat and pain-sensing nociceptors, which are further defined by their expression of neuropeptides, including CGRP (19,28), a potent vasodilator implicated in neurogenic inflammation (29,30). Consistent with the idea that E2 induces nociceptor sprouting, CGRP-ir neurons in vitro show increased neuritogenesis comparable to that of peripherin-ir neurons (14). E2 also affects this population of axons in vivo because CGRP-ir fiber density increases in cutaneous, glandular, and mesenteric targets of adult female rats after 7-d E2 treatment (18,31). Although it is unclear whether all subpopulations of peripherin-positive neurons respond comparably, our findings support the idea that E2 acts on presumptive DRG nociceptors to enhance axon outgrowth.
The possibility that E2 affects neuritogenesis by larger, peripherin-negative DRG neurons is an important consideration. ERs are present in DRG neurons of all sizes (32,33,34), and E2 elevation leads to expanded mechanoreceptive fields (35,36), which may suggest sprouting of large Aα/β-neurons as well. Results presented here argue against this. E2 did not affect axonal outgrowth of cultured NF200-ir neurons. Therefore, E2 selectively enhances neuritogenesis in small but not large DRG neurons.
Estrogen can influence genomic and nongenomic processes through several receptors, including the classical ERα and ERβ that bind to estrogen response elements and membrane-associated ER’s that act through ERK-MAPK signaling pathways. Although the full repertoire of E2 signaling mechanisms has not been defined, ERα and ERβ mRNA and protein are expressed by DRG neurons, making these receptors likely candidates mediating E2-induced sprouting. Indeed, the classical ER antagonist ICI blocked E2-induced sprouting in vitro, confirming participation of ERα and/or ERβ. Selective ER agonists provide further insight; the ERα agonist PPT was as effective as E2 in inducing neuritogenesis, whereas the ERβ agonist DPN was ineffective. This comports with prior findings that E2 enhances neuritogenesis by PC12 cells stably transfected with the gene encoding ERα (37). Thus, ERα activation is necessary and sufficient to induce DRG nociceptor sprouting.
The current studies show that E2 increases AT2 mRNA in cultured neonatal and intact adult DRG neurons, and elevated gene expression is accompanied by increased AT2 protein. E2’s effect on AT2 expression is selective, occurring in neurons with sizes and phenotypes similar to those undergoing enhanced neuritogenesis, and suggesting that E2-induced up-regulation of AT2 may play a causal role.
E2’s induction of AT2 expression in DRG is concordant with findings that E2 up-regulates AT2 in other tissues, including pituitary (38), myometrium (39), and kidney (40,41). Because the AT2 promoter apparently lacks canonical ER response elements, E2 likely acts through alternative signaling pathways, possibly involving activator protein-1 (40,42).
AT2 has been implicated in both axon regeneration and sprouting. DRG AT2 mRNA and protein are persistently up-regulated after sciatic nerve crush (43), and AT2 activation by ANGII elicits de novo neuritogenesis by PC12 and NG108–15 cells (22,26,44). AT2, a G protein-coupled receptor, increases intracellular signaling molecules implicated in axon outgrowth, including ERK (45), protein phosphatase 2A (46), nitric oxide, and cyclic GMP (22,47,48). Thus, AT2 up-regulation could explain E2’s effect on axon outgrowth.
Although AT2 has not been implicated previously in E2-induced neuritogenesis, our findings suggest an essential role. Receptor activation by ANGII promotes neuronal sprouting in culture, and AT2 has been consistently implicated. Selective blockade in the present study confirmed that AT2 is critical for ANGII-induced sprouting. More surprisingly, AT2 antagonism also eliminated E2-induced neuritogenesis. This provides evidence that AT2 is critical for axon outgrowth evoked not only by ANGII, but also by E2.
If AT2 mediates E2-induced neuritogenesis, then what ligand activates this receptor in vitro? ANGII is the classical AT2 ligand, so its presence within the culture may be inferred. However, the defined media used in these cultures lacked supplements that could provide extrinsic ANGII, raising the possibility that ANGII is synthesized in culture. ANGII synthesis occurs via a well-defined pathway. The α-glycoprotein AGN is cleaved by REN to yield ANGI, which is processed by ACE to biologically active ANGII. This pathway is best known with respect to cardiovascular homeostasis, in which circulating AGN is synthesized by the liver, REN by the kidney, and ACE by endothelial cells. More recently, RAS components have been identified in other tissues, including th heart, reproductive organs, digestive tract, and lymphatic tissues. In some cases such as vasculature and brain, all components of the RAS necessary for ANG formation are present, implying that ANGII can be synthesized locally through an intrinsic RAS (49).
We provide evidence that DRGs contain a complete intrinsic RAS. DRGs express mRNA encoding AGN, REN, and ACE, as well as AT2, and protein products of these genes are localized within small and medium-sized neurons. At least some components (AGN, REN) colocalize within single neurons, suggesting an autocrine pathway for ANGII formation. However, other cell types may participate in local ANGII generation because large neurons also express REN, and vasculature is a rich source of ACE. The functional importance of ANGII generated intrinsically is confirmed in DRG culture studies; E2 promotes neuritogenesis only when ACE is active. Although this intrinsic RAS is apparently unnecessary for basal outgrowth, it is essential for E2-enhanced neuritogenesis.
Gender and hormonal factors reportedly regulate RAS components, including AGN, REN, and ACE (50,51). However, in the DRG, E2 appears to have little overall effect on RAS gene expression, and very modest and inconsistent effects on the numbers of neurons expressing these proteins. Therefore, ANGII synthesis is apparently constitutive and relevant only when AT2 is up-regulated. Thus, E2 regulates DRG neuritogenesis at the level of the AT2 receptor rather than by affecting ligand synthesis.
This intrinsic ganglionic RAS poses interesting potential scenarios regarding regulation of nociceptive axon growth. AT2 and RAS expression are strongest during development (52); thus, DRG RAS may provide an autocrine mechanism whereby ANGII initiates neurite outgrowth from immature sensory neurons. Although caution must be exercised in extrapolating findings from cultured neonatal neurons to in vivo phenomena in adults, our findings suggest that AT2 may play a role in E2-induced target sensory hyperinnervation (18,31), and may involve extraganglionic ANGII. CGRP-ir hyperinnervation in vivo after E2 treatment is most robust in association with arteriolar blood vessels; these are particularly rich sources of ANGII because they provide ANGII from both circulation as well as the intrinsic vascular RAS (53). Thus, sensory neurons most susceptible to E2-induced remodeling may well be those with the greatest access to ANGII.
The finding that E2 promotes AT2-mediated sprouting of putative nociceptors has implications for female pain syndromes. Studies of human tissue show that at least some E2-related pain syndromes are associated with increased nociceptor innervation (9,10), and skin specimens from normal women are reported to have greater axon density than those of men (54). Collectively, these findings suggest that E2 may contribute to pain sensitivity in some conditions by promoting sprouting of DRG nociceptor axons as a result of E2-up-regulation of AT2 receptors on DRG unmyelinated neurons responding to autocrine or paracrine sources of ANGII. If so, pharmacological modulation of the RAS may present a strategy for altering the course of some E2-associated pain syndromes.
Acknowledgments
We thank Dr. T. Inagami (Vanderbilt University, Nashville, TN) for the generous gift of the renin antisera; Clark Bloomer and University of Kansas Medical Center School of Medicine Microarray Facility for assistance in conducting the microarray experiments; Sachin Mathur (Bioinformatics Core of the Kansas IDeA Network for Biomedical Research Excellence, Kansas City, KS) for assisting with data analysis; Dr. Dora Krizsan-Agbas for assistance with cell cultures; and Dr. Don Warn, Eric Grimes, and Phillip Shafer (Smith Center Integrative Imaging Core, Kansas City, KS) for assistance with image acquisition and analysis.
Footnotes
This work was supported by National Institute of Child Health and Human Development Grant RO1 HD049615 (to P.G.S.) with core support from P30 HD02528 and P20 RR016475.
Disclosure Statement: The authors have nothing to disclose.
First Published Online April 3, 2008
Abbreviations: ACE, Angiotensin converting enzyme; AGN, angiotensinogen; ANGI, angiotensin I; ANGII, angiotensin II; AT2, angiotensin II receptor type 2; CGRP, calcitonin gene-related peptide; DPN, diarylpropionitrile; DRG, dorsal root ganglion; EM, enalapril maleate; E2, estradiol; ER, estrogen receptor; GAPDH, glyceraldehyde phosphate dehydrogenase; ICI, ICI 182,780; -ir, immunoreactive; NF200, neurofilament 200; NGF, nerve growth factor; OVX, ovariectomized; PD, PD123,319; PPT, propyl pyrazole triol; RAS, renin-angiotensin system; REN, renin.
References
- Berkley KJ, Zalcman SS, Simon VR 2006 Sex and gender differences in pain and inflammation: a rapidly maturing field. Am J Physiol Regul Integr Comp Physiol 291:R241–R244 [DOI] [PubMed] [Google Scholar]
- Vallerand AH, Polomano RC 2000 The relationship of gender to pain. Pain Manag Nurs 1:8–15 [DOI] [PubMed] [Google Scholar]
- Fillingim RB 2000 Sex, gender, and pain: women and men really are different. Curr Rev Pain 4:24–30 [DOI] [PubMed] [Google Scholar]
- Fillingim RB, Ness TJ 2000 Sex-related hormonal influences on pain and analgesic responses. Neurosci Biobehav Rev 24:485–501 [DOI] [PubMed] [Google Scholar]
- Riley 3rd JL, Robinson ME, Wise EA, Price DD 1999 A meta-analytic review of pain perception across the menstrual cycle. Pain 81:225–235 [DOI] [PubMed] [Google Scholar]
- Brown MF, Hukkanen MV, McCarthy ID, Redfern DR, Batten JJ, Crock HV, Hughes SP, Polak JM 1997 Sensory and sympathetic innervation of the vertebral endplate in patients with degenerative disc disease. J Bone Joint Surg Br 79:147–153 [DOI] [PubMed] [Google Scholar]
- Alfredson H, Ohberg L, Forsgren S 2003 Is vasculo-neural ingrowth the cause of pain in chronic Achilles tendinosis? An investigation using ultrasonography and colour Doppler, immunohistochemistry, and diagnostic injections. Knee Surg Sports Traumatol Arthrosc 11:334–338 [DOI] [PubMed] [Google Scholar]
- Cain DM, Wacnik PW, Turner M, Wendelschafer-Crabb G, Kennedy WR, Wilcox GL, Simone DA 2001 Functional interactions between tumor and peripheral nerve: changes in excitability and morphology of primary afferent fibers in a murine model of cancer pain. J Neurosci 21:9367–9376 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gopinath P, Wan E, Holdcroft A, Facer P, Davis JB, Smith GD, Bountra C, Anand P 2005 Increased capsaicin receptor TRPV1 in skin nerve fibres and related vanilloid receptors TRPV3 and TRPV4 in keratinocytes in human breast pain. BMC Womens Health 5:2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pang X, Marchand J, Sant GR, Kream RM, Theoharides TC 1995 Increased number of substance P positive nerve fibres in interstitial cystitis. Br J Urol 75:744–750 [DOI] [PubMed] [Google Scholar]
- Bohm-Starke N, Hilliges M, Falconer C, Rylander E 1998 Increased intraepithelial innervation in women with vulvar vestibulitis syndrome. Gynecol Obstet Invest 46:256–260 [DOI] [PubMed] [Google Scholar]
- Tanzer L, Jones KJ 1997 Gonadal steroid regulation of hamster facial nerve regeneration: effects of dihydrotestosterone and estradiol. Exp Neurol 146:258–264 [DOI] [PubMed] [Google Scholar]
- Ahmed Y, Lin DL, Ferguson C, Esparza N, Damaser MS 2006 Effect of estrogen on urethral function and nerve regeneration following pudendal nerve crush in the female rat. J Urol 175:1948–1952 [DOI] [PubMed] [Google Scholar]
- Blacklock AD, Johnson MS, Krizsan-Agbas D, Smith PG 2005 Estrogen increases sensory nociceptor neuritogenesis in vitro by a direct, nerve growth factor-independent mechanism. Eur J Neurosci 21:2320–2328 [DOI] [PubMed] [Google Scholar]
- Fan X, Kim HJ, Warner M, Gustafsson JA 2007 Estrogen receptor β is essential for sprouting of nociceptive primary afferents and for morphogenesis and maintenance of the dorsal horn interneurons. Proc Natl Acad Sci USA 104:13696–13701 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patrone C, Andersson S, Korhonen L, Lindholm D 1999 Estrogen receptor-dependent regulation of sensory neuron survival in developing dorsal root ganglion. Proc Natl Acad Sci USA 96:10905–10910 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smyth GK 2004 Linear models and empirical bayes methods for assessing differential expression in microarray experiments. Stat Appl Genet Mol Biol 3:Article3 [DOI] [PubMed] [Google Scholar]
- Blacklock AD, Smith PG 2004 Estrogen increases calcitonin gene-related peptide-immunoreactive sensory innervation of rat mammary gland. J Neurobiol 59:192–204 [DOI] [PubMed] [Google Scholar]
- Goldstein ME, House SB, Gainer H 1991 NF-L and peripherin immunoreactivities define distinct classes of rat sensory ganglion cells. J Neurosci Res 30:92–104 [DOI] [PubMed] [Google Scholar]
- Ferri GL, Sabani A, Abelli L, Polak JM, Dahl D, Portier MM 1990 Neuronal intermediate filaments in rat dorsal root ganglia: differential distribution of peripherin and neurofilament protein immunoreactivity and effect of capsaicin. Brain Res 515:331–335 [DOI] [PubMed] [Google Scholar]
- Heldring N, Pike A, Andersson S, Matthews J, Cheng G, Hartman J, Tujague M, Strom A, Treuter E, Warner M, Gustafsson JA 2007 Estrogen receptors: how do they signal and what are their targets. Physiol Rev 87:905–931 [DOI] [PubMed] [Google Scholar]
- Cote F, Do TH, Laflamme L, Gallo JM, Gallo-Payet N 1999 Activation of the AT(2) receptor of angiotensin II induces neurite outgrowth and cell migration in microexplant cultures of the cerebellum. J Biol Chem 274:31686–31692 [DOI] [PubMed] [Google Scholar]
- Lucius R, Gallinat S, Rosenstiel P, Herdegen T, Sievers J, Unger T 1998 The angiotensin II type 2 (AT2) receptor promotes axonal regeneration in the optic nerve of adult rats. J Exp Med 188:661–670 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reinecke K, Lucius R, Reinecke A, Rickert U, Herdegen T, Unger T 2003 Angiotensin II accelerates functional recovery in the rat sciatic nerve in vivo: role of the AT2 receptor and the transcription factor NF-κB. FASEB J 17:2094–2096 [DOI] [PubMed] [Google Scholar]
- Gendron L, Payet MD, Gallo-Payet N 2003 The angiotensin type 2 receptor of angiotensin II and neuronal differentiation: from observations to mechanisms. J Mol Endocrinol 31:359–372 [DOI] [PubMed] [Google Scholar]
- Stroth U, Meffert S, Gallinat S, Unger T 1998 Angiotensin II and NGF differentially influence microtubule proteins in PC12W cells: role of the AT2 receptor. Brain Res Mol Brain Res 53:187–195 [DOI] [PubMed] [Google Scholar]
- Laflamme L, Gasparo M, Gallo JM, Payet MD, Gallo-Payet N 1996 Angiotensin II induction of neurite outgrowth by AT2 receptors in NG108–15 cells. Effect counteracted by the AT1 receptors. J Biol Chem 271:22729–22735 [DOI] [PubMed] [Google Scholar]
- Hiruma H, Saito A, Ichikawa T, Kiriyama Y, Hoka S, Kusakabe T, Kobayashi H, Kawakami T 2000 Effects of substance P and calcitonin gene-related peptide on axonal transport in isolated and cultured adult mouse dorsal root ganglion neurons. Brain Res 883:184–191 [DOI] [PubMed] [Google Scholar]
- Brack A, Rittner HL, Stein C 2004 Neurogenic painful inflammation. Curr Opin Anaesthesiol 17:461–464 [DOI] [PubMed] [Google Scholar]
- van Rossum D, Hanisch UK, Quirion R 1997 Neuroanatomical localization, pharmacological characterization and functions of CGRP, related peptides and their receptors. Neurosci Biobehav Rev 21:649–678 [DOI] [PubMed] [Google Scholar]
- Blacklock AD, Cauveren JA, Smith PG 2004 Estrogen selectively increases sensory nociceptor innervation of arterioles in the female rat. Brain Res 1018:55–65 [DOI] [PubMed] [Google Scholar]
- Yang Y, Ozawa H, Lu H, Yuri K, Hayashi S, Nihonyanagi K, Kawata M 1998 Immunocytochemical analysis of sex differences in calcitonin gene-related peptide in the rat dorsal root ganglion, with special reference to estrogen and its receptor. Brain Res 791:35–42 [DOI] [PubMed] [Google Scholar]
- Taleghany N, Sarajari S, DonCarlos LL, Gollapudi L, Oblinger MM 1999 Differential expression of estrogen receptor α and β in rat dorsal root ganglion neurons. J Neurosci Res 57:603–615 [PubMed] [Google Scholar]
- Sohrabji F, Miranda RC, Toran-Allerand CD 1994 Estrogen differentially regulates estrogen and nerve growth factor receptor mRNAs in adult sensory neurons. J Neurosci 14:459–471 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bereiter DA, Stanford LR, Barker DJ 1980 Hormone-induced enlargement of receptive fields in trigeminal mechanoreceptive neurons. II. Possible mechanisms. Brain Res 184:411–423 [DOI] [PubMed] [Google Scholar]
- Bradshaw HB, Berkley KJ 2000 Estrous changes in responses of rat gracile nucleus neurons to stimulation of skin and pelvic viscera. J Neurosci 20:7722–7727 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gollapudi L, Oblinger MM 2001 Estrogen effects on neurite outgrowth and cytoskeletal gene expression in ERα-transfected PC12 cell lines. Exp Neurol 171:308–316 [DOI] [PubMed] [Google Scholar]
- Suarez C, Diaz-Torga G, Gonzalez-Iglesias A, Cristina C, Becu-Villalobos D 2004 Upregulation of angiotensin II type 2 receptor expression in estrogen-induced pituitary hyperplasia. Am J Physiol Endocrinol Metab 286:E786–E794 [DOI] [PubMed] [Google Scholar]
- Mancina R, Susini T, Renzetti A, Forti G, Razzoli E, Serio M, Maggi M 1996 Sex steroid modulation of AT2 receptors in human myometrium. J Clin Endocrinol Metab 81:1753–1757 [DOI] [PubMed] [Google Scholar]
- Armando I, Jezova M, Juorio AV, Terron JA, Falcon-Neri A, Semino-Mora C, Imboden H, Saavedra JM 2002 Estrogen upregulates renal angiotensin II AT(2) receptors. Am J Physiol Renal Physiol 283:F934–F943 [DOI] [PubMed] [Google Scholar]
- Baiardi G, Macova M, Armando I, Ando H, Tyurmin D, Saavedra JM 2005 Estrogen upregulates renal angiotensin II AT1 and AT2 receptors in the rat. Regul Pept 124:7–17 [DOI] [PubMed] [Google Scholar]
- Ichiki T, Inagami T 1995 Transcriptional regulation of the mouse angiotensin II type 2 receptor gene. Hypertension 25:720–725 [DOI] [PubMed] [Google Scholar]
- Gallinat S, Yu M, Dorst A, Unger T, Herdegen T 1998 Sciatic nerve transection evokes lasting up-regulation of angiotensin AT2 and AT1 receptor mRNA in adult rat dorsal root ganglia and sciatic nerves. Brain Res Mol Brain Res 57:111–122 [DOI] [PubMed] [Google Scholar]
- Gendron L, Cote F, Payet MD, Gallo-Payet N 2002 Nitric oxide and cyclic GMP are involved in angiotensin II AT(2) receptor effects on neurite outgrowth in NG108–15 cells. Neuroendocrinology 75:70–81 [DOI] [PubMed] [Google Scholar]
- Steckelings UM, Czarnetzki BM 1995 The renin-angiotensin-system in the skin. Evidence for its presence and possible functional implications. Exp Dermatol 4:329–334 [DOI] [PubMed] [Google Scholar]
- Huang XC, Sumners C, Richards EM 1996 Angiotensin II stimulates protein phosphatase 2A activity in cultured neuronal cells via type 2 receptors in a pertussis toxin sensitive fashion. Adv Exp Med Biol 396:209–215 [DOI] [PubMed] [Google Scholar]
- Zhao Y, Biermann T, Luther C, Unger T, Culman J, Gohlke P 2003 Contribution of bradykinin and nitric oxide to AT2 receptor-mediated differentiation in PC12 W cells. J Neurochem 85:759–767 [DOI] [PubMed] [Google Scholar]
- Gendron L, Oligny JF, Payet MD, Gallo-Payet N 2003 Cyclic AMP-independent involvement of Rap1/B-Raf in the angiotensin II AT2 receptor signaling pathway in NG108–15 cells. J Biol Chem 278:3606–3614 [DOI] [PubMed] [Google Scholar]
- Paul M, Poyan Mehr A, Kreutz R 2006 Physiology of local renin-angiotensin systems. Physiol Rev 86:747–803 [DOI] [PubMed] [Google Scholar]
- Fischer M, Baessler A, Schunkert H 2002 Renin angiotensin system and gender differences in the cardiovascular system. Cardiovasc Res 53:672–677 [DOI] [PubMed] [Google Scholar]
- Greenland KJ, Sernia C 2004 Oestrogenic regulation of brain angiotensinogen. J Neuroendocrinol 16:508–515 [DOI] [PubMed] [Google Scholar]
- Lucius R, Gallinat S, Busche S, Rosenstiel P, Unger T 1999 Beyond blood pressure: new roles for angiotensin II. Cell Mol Life Sci 56:1008–1019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muller DN, Luft FC 1998 The renin-angiotensin system in the vessel wall. Basic Res Cardiol 93(Suppl 2):7–14 [DOI] [PubMed] [Google Scholar]
- Mowlavi A, Cooney D, Febus L, Khosraviani A, Wilhelmi BJ, Akers G 2005 Increased cutaneous nerve fibers in female specimens. Plast Reconstr Surg 116:1407–1410 [DOI] [PubMed] [Google Scholar]