Abstract
Signal transducer and activator of transcription (Stat)-3 signals mediate many of the metabolic effects of the fat cell-derived hormone, leptin. In mice, brain-specific depletion of either the long form of the leptin receptor (Lepr) or Stat3 results in comparable obese phenotypes as does replacement of Lepr with an altered leptin receptor locus that codes for a Lepr unable to interact with Stat3. Among the multiple brain regions containing leptin-sensitive Stat3 sites, cells expressing feeding-related neuropeptides in the arcuate nucleus of the hypothalamus have received much of the focus. To determine the contribution to energy homeostasis of Stat3 expressed in agouti-related protein (Agrp)/neuropeptide Y (Npy) arcuate neurons, Stat3 was deleted specifically from these cells, and several metabolic indices were measured. It was found that deletion of Stat3 from Agrp/Npy neurons resulted in modest weight gain that was accounted for by increased adiposity. Agrp/Stat3-deficient mice also showed hyperleptinemia, and high-fat diet-induced hyperinsulinemia. Stat3 deletion in Agrp/Npy neurons also resulted in altered hypothalamic gene expression indicated by increased Npy mRNA and decreased induction of suppressor of cytokine signaling-3 in response to leptin. Agrp mRNA levels in the fed or fasted state were unaffected. Behaviorally, mice without Stat3 in Agrp/Npy neurons were mildly hyperphagic and hyporesponsive to leptin. We conclude that Stat3 in Agrp/Npy neurons is required for normal energy homeostasis, but Stat3 signaling in other brain areas also contributes to the regulation of energy homeostasis.
LEPTIN IS A HORMONE that is synthesized in fat cells. Loss-of-function mutations in the genes for leptin or its receptor are associated with massive obesity in man or rodents (1). In mice, the obesity phenotype was reproduced when the leptin receptor gene (Lepr) was deleted specifically from neurons, thereby implicating the brain as a major leptin target organ (2). Lepr is expressed in a number of brain regions and is concentrated in hypothalamic, midbrain, and brain stem regions involved in energy homeostasis (3,4,5,6).
Lepr is a member of the class I cytokine receptor family and signals through activation of the Janus tyrosine kinase-2 bound to the cytoplasmic tail of the receptor (7). Activation of Janus tyrosine kinase-2 stimulates activity in several signaling pathways, the best characterized of these being activation of the transcription factor, signal transducer and activator of transcription (Stat)-3. The major metabolic effects of leptin have been shown to be mediated by Stat3 because specific neuronal deletion of Stat3 recapitulated the obesity phenotype seen in Lepr-deficient (db/db) mice (8). This conclusion was supported by knocking-in mutated Lepr alleles that prevented Lepr-Stat3 interactions and reproduced much of the db/db phenotype (9).
Initial models of a hypothalamic neural circuit for energy homeostasis highlighted Lepr expression in two distinct neuronal subtypes within the arcuate nucleus. Neurons expressing proopiomelanocortin (Pomc) where shown to be stimulated to synthesize Pomc and increase firing in response to leptin (10,11). Conversely, neighboring neurons expressing agouti-related peptide (Agrp) and neuropeptide Y (Npy) were shown to decrease the synthesis of these neuropeptides and inhibit firing in response to leptin (12). The processing of Pomc to melanocortin products provides agonist molecules for melanocortin receptors (MCRs), which, when activated, suppress weight gain. Agrp functions as an antagonist at these receptors. Both Agrp and Npy have been shown to stimulate feeding when injected into the brains of rodents (13,14). Both Pomc and Agrp/Npy neurons send projections to extraarcuate sites expressing MCRs, and the differential action of leptin on the Pomc and Agrp/Npy neurons was seen to mediate leptin’s metabolic effects by promoting and inhibiting delivery of agonist and antagonist molecules, respectively, to MCRs. For example, fasting-induced stimulation of appetite was thought to result from fasting-induced suppression of circulating leptin with consequent increases in Agrp and Npy expression and decreased Pomc expression.
The above model has been tested using cell-specific deletions of Lepr or Stat3 from Pomc and Agrp/Npy neurons. Cell-specific loss of Lepr from Pomc neurons resulted in a modest obesity phenotype with decreased transcript levels of Pomc and Agrp with no change in mRNA for Npy (15). Cell-specific loss of Stat3 in Pomc neurons also resulted in a modest obesity and decreased Pomc mRNA (16). In a previous study (17), we reported the surprising result of normal Agrp expression in both fed and fasted states in Agrp/Npy neurons lacking Stat3. In the present study, we report that despite normal Agrp transcript levels, Stat3 in Agrp/Npy neurons is required for normal body weight regulation and that loss of Stat3 from these cells resulted in elevated Npy mRNA, modest hyperphagia and obesity, hyperleptinemia, and reduced sensitivity to leptin.
Materials and Methods
Animal care
All animal procedures in this study were approved by the Wayne State University Institutional Animal Care Use Committee. Mice were maintained on 12-h light, 12-h dark cycles with normal chow and water ad libitum.
BAC AgrpCre mouse
Methods and results on the further characterization of the AgrpCre transgenic mouse are presented in supplemental data files published on The Endocrine Society’s Journals Online web site at http://endo.endojournals.org.
Metabolic profiling
Locomotor activity, calorimetric, and body composition measurements were performed at the Mouse Metabolic Phenotyping Center at the University of Washington. Eight- to 12-wk-old CON and DEL littermate controls were individually housed and acclimated to metabolic cages for 3 d. Activity and calorimetric measurements were continuously recorded over a 24-h period during which food was available ad libitum. Determinations of body lean and fat mass were made in conscious mice using quantitative magnetic resonance (EchoMRI 3-in-1 machine whole body composition analyzer; Echo Medical Systems, Houston, TX). Locomotor activity was assessed by the infrared beam break method using an Opto-Varimetrix-3 sensor system, whereas food and water intake were measured with the Feed-Scale System (Columbus Instruments, Columbus, OH). Indirect calorimetry was performed with a computer-controlled open circuit calorimetry system (Oxymax; Columbus Instruments). Rates of oxygen consumption (VO2) were determined at 6-min intervals and were normalized to lean body mass. The wet weights of epididymal fat pads were recorded. Plasma leptin levels were determined using a mouse leptin ELISA kit (R&D Systems, Minneapolis, MN). Plasma insulin was determined by a rat/mouse insulin ELISA kit (Linco Research, St. Charles, MO). Plasma corticosterone was measured by ELISA using the Octeia corticosterone kit (Immunodiagnostics, Fountain Hills, AZ). Blood glucose levels were determined photometrically using the HemoCue B-glucose kit (HemoCue, Mission Viejo, CA). Mice placed on a high-fat diet were fed a 60 kilocalories % (kcal%) fat diet (Research Diets, New Brunswick, NJ).
Quantitative RT-PCR (QRT-PCR)
Real-time PCR was performed using an iCycler (Bio-Rad, Hercules, CA) and the manufacturer’s SYBR green protocol. The assays were run in 96-well format. Expression of a given gene was determined in triplicate. Expression of the reference gene, mouse β-actin, was determined for each plate in triplicate. cDNA samples were evaluated for DNA contamination by performing QRT-PCR using an intron-spanning primer pair for β-actin and running an ethidium bromide-stained agarose gel of the products. For QRT-PCR, 2 μl of cDNA were added to 1 μl of primers (2.5 μm each), 12 μl of double-distilled H2O, and 15 μl of iQTM SYBR green supermix (Bio-Rad). The reaction was initiated by denaturation at 95 C for 3 min followed by 40 cycles of 15 sec at 95 C, 30 sec at 58 C, and 30 sec at 72 C. PCR primers were: Npy forward, 5′-CTGACCCTCGCTCTATCTCTG-3′, reverse, 5′-AGTATCTGGCCATGTCCTCTG-3′ (accession no. NM 023456.2); Pomc forward, 5′-CCCAAGGACAAGCGTTACGG-3′, reverse, 5′-GTGCGCGTTCTTGATGATGG-3′ (accession no. NM 008895.3); Agrp forward, 5′-TTGTGTTCTGCTGTTGGCACT-3′, reverse, 5′-AGCAAAAGGCATTGA AGAAGC-3′ (accession no. NM 007427.2); β-actin forward, 5′-CAACGAGCGGTTCCGATG-3′, reverse, 5′-CACTGTGTTGGCATAGAGG-3′ (accession no. NM 007393.2); suppressor of cytokine signaling (Socs) forward, 5′-AGAAGATTCCGCTGGTACTG-3′, reverse, 5′-GGGTCACTTTCTCATAGGAG-3′ (accession no. NM 007707.2).
Melt-curve analysis was performed immediately after the amplification to test for primer-dimer formation using the following conditions: 1 min denaturation at 95 C, 1 min annealing at 55 C, 80 cycles of 0.5-C increments (10 sec each) beginning at 55 C. Melt-curve results plotting −d(fluorescence)/dT vs. temperature (the negative rate change in fluorescence as a function of temperature) were captured and plotted by the iCycler iQ data analysis software module. To determine primer pair PCR amplification efficiency, cDNA was made from total RNA of whole-mouse brain (CLONTECH BD Biosciences, Palo Alto, CA) using the Omniscript reverse transcription kit (QIAGEN, Valencia, CA) and diluted to final concentrations of 0.005, 0.05, 0.5, 5, and 50 ng for QRT-PCR as described above. Each primer pair was run in triplicate at each input concentration. Primer concentrations were those used for QRT-PCR described above. Plots of threshold cycle (CT) vs. input concentration were determined and the correlation coefficients, slopes, and efficiencies calculated by the iCycler iQ data analysis software module. The efficiency of PCR was calculated using the equation E = 10−1/s − 1 where s is the slope of the log input concentration vs. ΔCT (18).
Relative expression was calculated using the 2-ΔΔCT method (19) by determining the Δ cycle threshold (ΔCT) as the CT of the gene of interest CT − the CT of the housekeeping gene CT. SΔCT-CΔCT was the difference between the sample ΔCT and the control ΔCT, and relative expression was calculated as 2-(SΔCT-CΔCT).
Immunohistochemistry
Mice were killed by anesthesia and transcardial perfusion of saline followed by 4% paraformaldehyde. The brains were excised and placed in ice cold 4% paraformaldehyde for 4 h and then in 20% sucrose at 4 C overnight.
For the detection of c-fos like immunoreactivity (CFLIR) by immunofluorescence, mice were killed and the brains removed and sectioned as described above. CFLIR was detected using a rabbit anti-c-fos primary antibody (no. PC05; Oncogene Research Products, San Diego, CA) at 1:500 dilution and an Alexa Fluor 568 goat anitrabbit secondary antibody (Molecular Probes, Eugene, OR). The Alexa 568 and enhanced green fluorescent protein (Egfp) fluorophores were visualized and acquired using an IX70 inverted Olympus microscope (Olympus, Mellville, NY) with mercury arc illumination with a standard filter cube and a KP-D590P charge-coupled device color camera (Hitachi, Tokyo, Japan).
CFLIR fluorescence intensity was determined by acquiring ×20 images with an IX-81 microscope (Olympus, Tokyo, Japan) equipped with automated filter controls and an ORCA cooled charge-coupled device camera (Hamamatsu, Bridgewater, NJ). The images were analyzed using Image-Pro Plus 4.5 (Media Cybernetics, Silver Spring, MD) to count and identify cells of above-background fluorescence intensity (FI) and sum the intensities across pixels for each cell within an area of interest (the arcuate nucleus). FI across cells were summed to determine total FI from all cells within the area of interest. Image-Pro Plus (Media Cybernetics) was also used to generate a pseudocolor surface plot to obtain a three-dimensional representation of the intensities of Alexa 568 fluorescence with warmer hues representing pixels of higher FI.
Leptin sensitivity
Singly caged, adult (20 wk) CON and DEL mice, monitored daily for food intake and body weight, were challenged with leptin (2.5 mg/kg, ip, twice daily, at 1000 and 1630 h) for 4 d. Daily body weight and food intake were measured for 18 d, and the following day mice were killed and whole hypothalami and fat pads removed for analysis. Hypothalami were dissected by placing the brain ventral side up in an ice-cooled stainless steel brain matrix with 1-mm coronal slice intervals (Zivic Instruments, Pittsburgh, PA). A 2-mm-thick coronal slice was cut just caudal to the optic chiasm, placed on a cooled glass dissecting dish, and trimmed with cuts in the sagittal plane at 1 mm on either side of the midline and a cut in the horizontal plane 1 mm from the base of the brain.
The effects of leptin on neuropeptide gene expression was determined in another experiment in which 24-wk-old CON (n = 9) and DEL (n = 9) mice had food removed on d 1. On the same day, mice from each group were injected with 5 mg/kg ip leptin (n = 5/group) or vehicle (n = 4/group) at 1000 and 1600 h. On the following day, the same mice were injected again with leptin (5 mg/kg ip) or vehicle at 900 h and were killed 1 h later. The brains were removed and whole hypothalami dissected for QRT-PCR analysis of neuropeptides and Socs-3 transcript levels.
Results
All results are from male mice unless otherwise indicated.
Loss of Stat3 in Agrp/Npy neurons leads to increased body weight and adiposity and hyperleptinemia
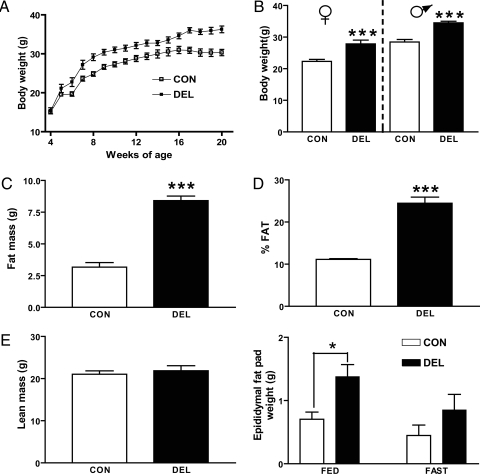
Targeted deletion of Stat3 from brain and undefined hypothalamic neurons has been shown to promote increased body weight and adiposity (8,20). In this study, DEL mice showed a 22% increase relative to CON mice by 16 wk of age (Fig. 1, A and B). This effect was also apparent in females (24% increase) (Fig. 1B). AgrpCre/+ mice weighed the same as CON mice at all ages. Increased body mass was accounted for by increased fat mass (Fig. 1C) with greater than 2-fold increase in adiposity (Fig. 1D) and no change in lean body mass (Fig. 1E). Epididymal fat pad weight was doubled in DEL mice in both the fed and fasted states but was not increased in AgrpCre/+ mice.
Figure 1.
Loss of Stat3 from Agrp/Npy neurons resulted in increased adiposity. A, CON mice (n = 6–22) and DEL littermates (n = 5–21) were weighed weekly for 20 wk. Two-way ANOVA indicated statistically significant effects of both time and genotype (P < 0.001) and a significant interaction between these factors (P < 0.05). B, Body weights of 12-wk-old males (n = 5/group) and females (n = 8–11/group) showed DEL mice to weigh more (P < 0.001) than littermate controls. Twelve-week-old males (n = 5/group) were analyzed for body fat (C and D) and lean mass (E) as described in Materials and Methods. F, Weights of epididymal fat pads from 20-wk-old fed or fasted (48 h) mice (n = 4 mice/group). Statistical analysis by two-way ANOVA indicates significant effects of genotype (P < 0.01) and fasting (P < 0.05) but no interaction effect. Values are means ± sem. *, Statistical significance at P < 0.05; ***, P < 0.001 by two-tailed unpaired t test.
There were no detectable differences between CON and DEL mice measures of VO2, respiratory exchange ratio (CO2 production to VO2), heat production (kilocalories per hour), or locomotor activity (data not shown).
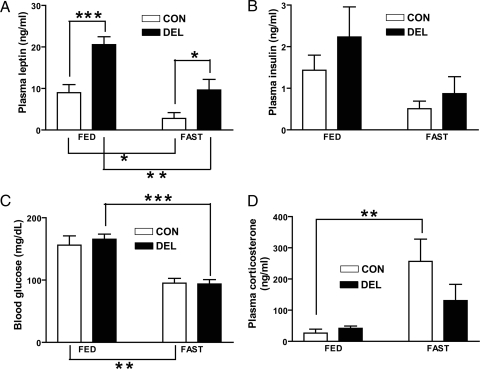
Plasma levels of the fat-derived hormone, leptin, have been shown to correlate with adiposity (21), and this relationship was confirmed in DEL mice in which plasma leptin was increased 2-fold or greater in both fed and fasted states (Fig. 2, A). Plasma insulin and corticosterone levels and blood glucose all varied by metabolic state (fed vs. fasting) but did not differ by genotype (Fig. 2, B–D).
Figure 2.
Hormones and glucose levels of 20-wk-old fed or fasted (48 h) CON and DEL littermates (n = 4–6/group). Values are means ± sem. *, P < 0.05; **, P < 0.01; ***, P < 0.001 by two-tailed unpaired t test between indicated comparisons. For all measures, a two-way ANOVA indicated a significant fasting effect with no interaction. Only leptin showed a significant (P < 0.001) effect of genotype.
Sensitivity to a high-fat diet (HFD)
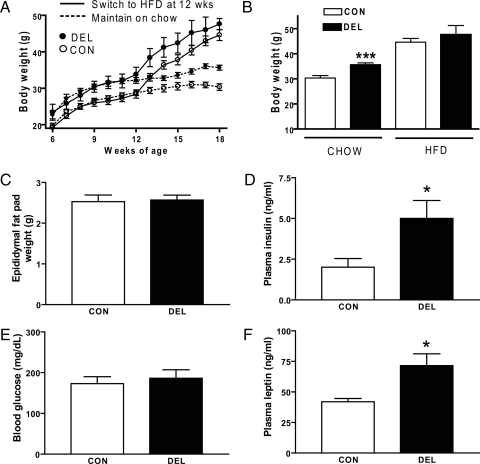
Twelve-week-old CON and DEL mice were placed on a 60 kcal%HFD for 6 wk, then fasted overnight, killed the next day, and blood and epididymal fat pads collected. Body weights were measured from 6 wk of age. The HFD increased body weight in both groups (Fig. 3A) over those kept on normal chow (compare with Fig. 1A) and, if anything, reduced the body weight difference between the genotypes. Consumption of HFD also increased epididymal fat pad weight for both groups (compare Fig. 1F with 3B) and prevented the genotypic difference in fat pad weight seen on normal chow. Plasma insulin, leptin, and blood glucose were elevated by HFD (compare fasting levels of Fig. 1, A–C, with Fig. 3, C–E) in both groups. DEL mice exhibited both hyperinsulinemia and hyperleptinemia relative to controls.
Figure 3.
Effect of 6-wk HFD on metabolic indices of CON and DEL littermates. Mice were 18 wk old and fasted for 24 h before they were killed (n = 5/group). A, Two-way ANOVA of mice switched to HFD at wk 12 indicated significant (P < 0.0001) effects of both age and genotype on body weight. Data for mice maintained on chow for 18 wk are from Fig. 2A and are presented here for comparison with mice switched to HFD at wk 12. B, Body weights for mice at 18 wk of age either maintained on chow (data from Fig 2A) or switched from chow to HFD at 12 wk of age. C–F, Additional metabolic indices of mice on HFD. Statistical analyses for B–F are by two-tailed, unpaired t tests: ***, P < 0.001; *, P < 0.05; values are means ± sem.
Fasting-induced CFLIR is reduced in Stat3-deleted Agrp/Npy neurons
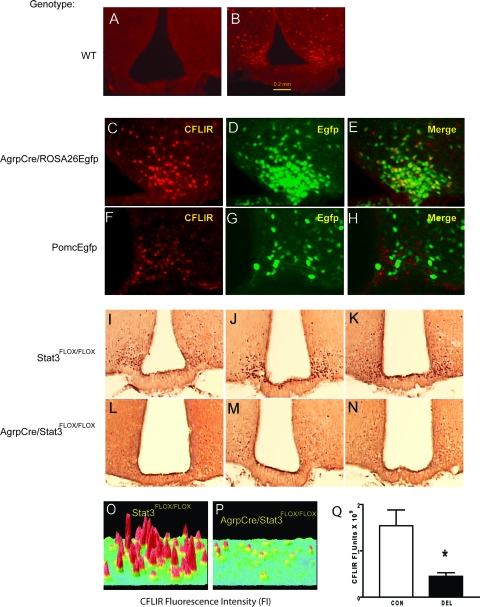
For a 48-h fast, food was removed from the cages between 1000 and 1100 h, and the mice were killed 48 h later. Water was continuously available. A 48-h fast induced CFLIR in the arcuate nucleus (Fig. 4, A and B). Using AgrpCre/ROSA26Egfp and PomcEgfp (10) mice, it was shown that fasting-induced CFLIR was restricted to Agrp/Npy neurons (Fig. 4, C–H). To determine whether Stat3 deletion from these neurons affected fasting-induced CFLIR, CON and DEL mice were fasted for 48 h and their arcuate nuclei immunostained for CFLIR. As shown in Fig. 4, I–Q, Agrp/Npy neurons without Stat3 showed a marked reduction of CFLIR in response to fasting.
Figure 4.
Fasting-induced rise in CFLIR was decreased in Agrp/Npy neurons in DEL mice. CFLIR by immunofluorescence in the arcuate nucleus in the fed state (A) and after 48 h fasting (B). Fasting-induced CFLIR (C) and green fluorescent cells expressing Egfp (D) in AgrpCre/ROSA26Egfp mice are shown. E, Colocalization of CFLIR and Egfp in AgrpCre/ROSA26Egfp mice. Fasting-induced CFLIR (F) and green fluorescent cells (G) expressing Egfp in PomcEgfp mice. H, CFLIR and Egfp in PomcEgfp mice. I, CFLIR by avidin biotin complex staining in the arcuate of 48-h fasted 8- to 12-wk-old mice was greater in CON mice (I–K) than DEL littermates (L–N). O and P, Surface plots of CFLIR by immunofluorescence after 48 h fasting in CON (O) and DEL littermates (P). Q, Means ± sem of summed FI units for fasting-induced CFLIR by immunofluorescence in arcuate nucleus of CON and DEL littermates (n = 4/group).
Loss of Stat3 in Agrp/Npy neurons results in decreased sensitivity to leptin
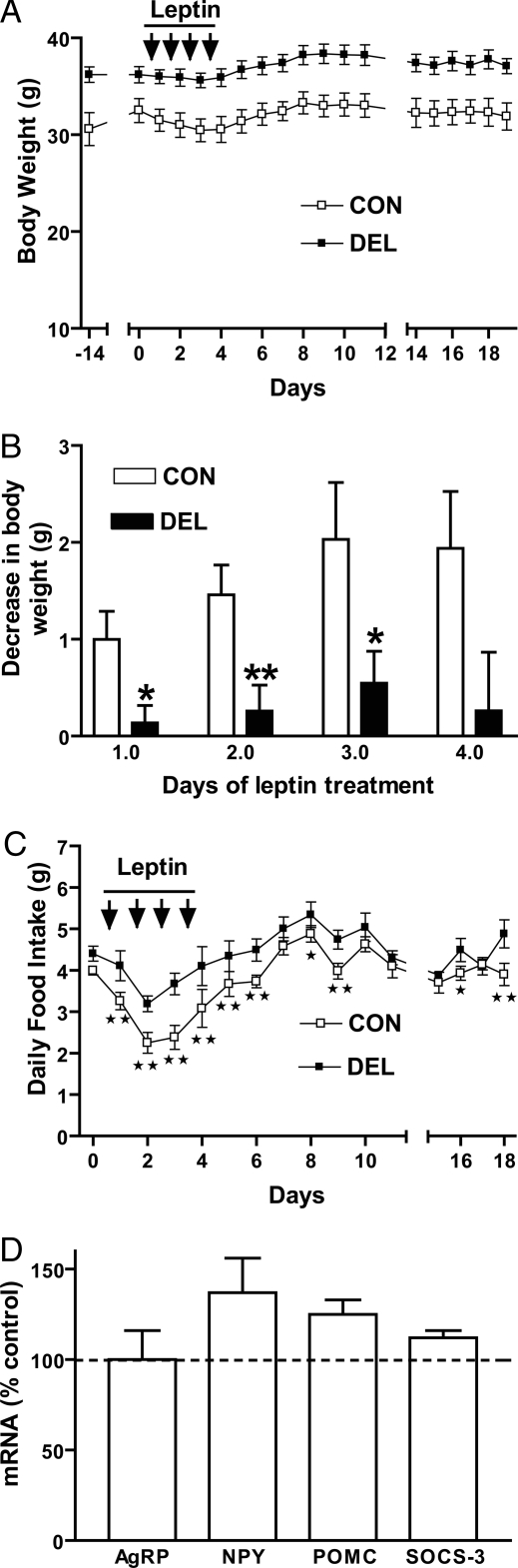
To determine whether Stat3 deletion from Agrp/Npy neurons would affect responses to leptin, adult mice were injected with leptin twice a day for 4 d, and body weight and food intake were measured daily. In response to leptin, DEL mice lost less weight and decreased food intake less than controls (Fig. 5, A–C). Also, during the 15-d postleptin treatment period, DEL mice exhibited a small (12%) but statistically significant difference in cumulative food intake, compared with CON mice (CON, 61.1 ± 1.7 g vs. DEL, 68.6 ± 1.5 g; P < 0.01 by two-tailed unpaired t test). Postmortem analysis of relative transcript levels in these mice indicated no significant differences, although mRNA for Npy showed a tendency to be elevated in DEL mice (Fig. 5, D). Weights of epididymal fat pads were also determined (CON = 0.56 ± 0.08 g, DEL = 1.33 ± 0.16 g; P < 0.01 by two-tailed, unpaired t test) and exhibited the genotypic differences seen previously (Fig. 1F).
Figure 5.
Effect of leptin on body weight (A and B) of 22-wk-old CON mice and DEL littermates (n = 5/group). Leptin was administered as described in the text. Two-way ANOVA of body weight from d 0 to d 19 indicated an effect of genotype (P < 0.0001). B, Body weight differences from d 0 were determined for the 4 d of leptin treatment and group differences tested by two-tailed unpaired t tests. C, Two-way ANOVA of food intake showed significant effects of both genotype (P < 0.0001) and time (P < 0.0001) with no interaction. Group differences in daily food intake were analyzed by two-tailed, unpaired t tests. Mice were killed after the last day of feeding measurements, and neuropeptide and Socs-3 transcripts from whole hypothalamus (D) were quantified. *, P < 0.05; **, P < 0.01. Values are means ± sem.
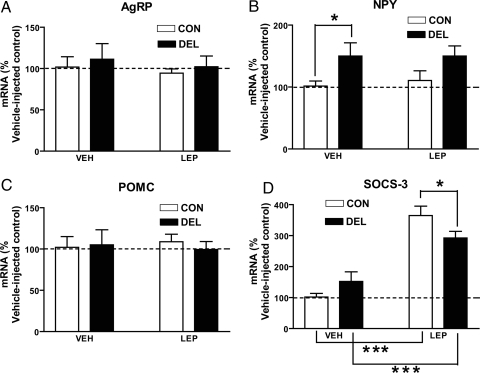
The effects of leptin on hypothalamic gene expression was determined in a separate experiment in which 24-wk-old CON (n = 9) and DEL (n = 9) mice were fasted and challenged with leptin or vehicle as described in Materials and Methods. As seen in Fig. 6, the leptin regimen was without effect on neuropeptide mRNA (Fig. 6, A–C). Npy was elevated in vehicle-injected DEL mice. In both groups, leptin treatment was associated with increased hypothalamic Socs-3 mRNA, although this response was smaller in leptin-injected DEL mice.
Figure 6.
Effect of leptin on whole hypothalamic neuropeptide and Socs-3 mRNA in 24-wk-old CON and DEL littermates. Twenty-four-week-old CON (n = 9) and DEL (n = 9) mice had food removed on d 1. On the same day, mice from each group were injected with 5 mg/kg ip leptin (n = 5/group) or vehicle (n = 4/group) at 1000 and 1600 h. On the following day, the same mice were injected again with leptin (5 mg/kg ip) or vehicle at 0900 h and were killed 1 h later. *, P < 0.05; ***, P < 0.001. Values are means ± sem.
Discussion
The brain-specific deletion of Stat3 in mice has been shown to produce a phenotype marked by massive obesity indistinguishable from mice (db/db) with loss-of-function mutations in Lepr (8). This phenotype is also reproduced in mice with a mutated knocked-in Lepr allele designed to prevent Lepr-Stat3 interactions (9). Given the known role of Stat3 in the Lepr signal transduction pathway, it would appear that normal energy homeostasis requires the expression of Stat3 in the brain.
Our previous work had shown that the AgrpCre/Stat3flox/flox (DEL) mice lack Stat3 in arcuate neurons that express Npy (17). In the present study, results with an Egfp reporter strains indicate that Cre recombinase expression was restricted to the arcuate nucleus (see supplemental data files).
Loss of Stat3 in Agrp/Npy neurons resulted in a 5- to 6-g weight gain (22–24% increase) in adult mice of both sexes. This modest increase was entirely accounted for by a greater than 2-fold increase in whole-body fat mass with no change in lean body mass. The increased adiposity was reflected by a doubling of epididymal fat pad mass and plasma leptin levels. On normal chow, blood glucose and insulin levels were not affected, which is consistent with Stat3-independent effects of leptin previously shown for glucose homeostasis (23). These changes were consistent with the presence of leptin resistance seen when mice are fed a HFD (24). A proposed mechanism for leptin resistance has focused on overstimulation of Lepr by high leptin levels and subsequent overproduction of a Stat3-inducible gene, Socs-3. Socs-3 has been shown to serve a negative regulatory function by limiting activation of Stat3 and possibly inhibiting other Lepr-activated signaling pathways (25). The absence of leptin-induced activation of Stat3 in Agrp/Npy neurons in DEL mice (17) would be expected to result in cell-specific leptin resistance in these cells because the metabolic actions of leptin are thought be mediated by Stat3 (8,9). When DEL mice were placed on a HFD, epididymal fat pad weight increased 2.5-fold and leptin levels rose by 6-fold, indicating that these mice were fully susceptible to further leptin resistance. Also, the fact that body weights of CON and DEL mice appear to converge on a HFD suggests that one consequence of high-fat feeding might be reduced Stat3 function in Agrp/Npy neurons. Previous work by others has shown reduced Stat3 activation within the arcuate nucleus of diet-induced obese mice (26). Taken together, results from DEL mice indicating modest weight gain on normal chow and susceptibility to further leptin resistance on a HFD are in keeping with other studies using cell-specific genetic dissection of leptin-regulated energy homeostatic pathways in the brain, namely that the system is distributed across multiple hypothalamic and extrahypothalamic loci (24,27).
Leptin has been shown to regulate the firing rate of target neurons in different loci and of differing neuronal phenotypes (10,12,28) and is thought to inhibit the activity of Agrp/Npy neurons (12,29). In neurons, c-fos functions as an activity-dependent immediate early gene, and c-fos expression has been used as an indicator of neuronal activity. The rise in c-fos expression in the arcuate nucleus during fasting has been interpreted as a sign of increased neuronal activity, reflecting a release from inhibition by leptin, because leptin levels fall during a fast (12).
Previous work by others has shown that leptin regulation of CFLIR in the arcuate appeared to be independent of Stat3 (30) by showing that CFLIR was not inappropriately induced in Agrp/Npy neurons in fed mice with mutated Lepr uncoupled from Stat3. In contrast, db/db mice with complete Lepr loss of function exhibit aberrant CFLIR expression in the fed state.
The decreased CFLIR induction in Agrp/Npy neurons in fasted DEL mice could reflect leptin-induced inhibition of neuronal activity given the persistent hyperleptinemia in these animals. This would imply that leptin effects on neuronal firing are Stat3 independent, which would agree with conclusions drawn in a recent paper (30), although it would not explain how the chronic inhibition of these orexigenic neurons would result in increased adiposity. On the other hand, chronic leptin resistance (see below) might uncouple CFLIR expression from changes in leptin levels associated with different metabolic states. Alternatively, diverse hormonal, metabolic, and synaptic inputs in addition to leptin have been shown to affect Agrp/Npy neuronal function, and one or more of these could be altered in DEL mice to reduce fasting-induced CFLIR in these cells (31,32,33,34,35).
When DEL mice were challenged with leptin, they lost less weight and decreased feeding less than controls. Furthermore, daily food intake measurements indicated occasional 24-h hyperphagia by the DEL mice. This decreased sensitivity to leptin was consistent with mice already exhibiting signs of leptin resistance, evidenced by increased adiposity and hyperleptinemia measured in other DEL mice. In previous work, we had shown a tendency for Npy to be elevated in DEL mice (17), and this same tendency was also apparent in these mice that were killed 2 wk after the last leptin injection. The decreased responsiveness to leptin could be a direct result of the loss of Stat3 in Agrp/Npy neurons or it could reflect the altered metabolic state of the DEL mice.
A separate experiment testing the effects of leptin on neuropeptide expression was unable to demonstrate any effect of peripheral leptin on hypothalamic neuropeptide expression. In vehicle-treated mice, Npy was significantly elevated in the DEL mice and again showed a tendency to be elevated in leptin-injected DEL mice. Overall, these results indicate that loss of Stat3 in Agrp/Npy neurons results in an up-regulation of Npy expression, and this could play a role in the modest hyperphagia seen in DEL mice. We attribute the lack of a leptin effect on neuropeptide expression either to strain differences or the age of the mice because we have found small but significant decreases in Npy and Agrp and increases in Pomc in younger mice of different strains (not shown). Alternatively, leptin effects on neuropeptide expression might have been seen if a different time point had been used. The leptin treatment appeared to effectively activate hypothalamic leptin receptors because in the same hypothalamic samples, leptin increased Socs-3 mRNA by 3.5-fold in CON mice and 1.9-fold in DEL mice. The blunted Socs-3 response in DEL mice could directly reflect the Stat3 deletion in Agrp/Npy neurons, or it could reflect a more general leptin resistance due to chronic hyperleptinemia or some other, presently unknown, consequence of obesity.
In previous work, we were surprised to see that loss of the Stat site in the Agrp locus prevented fasting-induced up-regulation of Agrp but that loss of Stat3 from Agrp/Npy neurons had no effect on Agrp expression in the fed or fasted states (17). This latter observation was again confirmed in the present study. Recent work demonstrating a requirement for the Foxo1 transcription factor in Agrp expression indicated an inhibitory role for Stat3 via transcriptional squelching (36). This mechanism would predict an up-regulation of Agrp in DEL mice in the fed state, although is it possible that this, but not all, actions of Stat3 could be replaced by Stat5,which has been recently shown to also be regulated by leptin in rat arcuate neurons (37). Regulation of Agrp and Npy expression is clearly complex and has been shown to also require signaling through the phosphatidylinositol-3 kinase pathway (38) and the brain-specific homeobox factor, Bsx (39).
We do not currently have a clear explanation for the modest obesity phenotype produced by Stat3 deletion in Agrp/Npy neurons. It is possible that small decreases in energy expenditure that were not detected in the metabolic profiling are responsible. It is likely that the occasional 24-h hyperphagia contributes to the phenotype, but to what extent remains to be determined. Also unclear is the relationship between the hyperphagia and elevated Npy expression. Based on work by others, we did not expect to see elevated Npy mRNA. In mice with a mutated Lepr unable to activate Stat3 (s/s mice), Agrp mRNA but not Npy mRNA was elevated, suggesting Stat3 regulation of Agrp but not Npy expression (9). These discrepant Agrp results can be explained by results from a recent work that shows that deletion of Stat3 specifically in Lepr-expressing cell leads does not result in elevated Agrp and Npy mRNA until the mice become severely obese (22), a state not reached in the modest obesity of DEL mice. It would appear that deletion of Stat3 in leptin-sensitive cells has metabolic effects that, in turn, alter the expression of hypothalamic neuropeptides involved in feeding. The severe obesity seen in the whole-animal (9, 40) or brain-specific (8) uncoupling of Stat3 from Lepr highlights the importance of other, extraarcuate Stat3-mediated pathways in the control of energy homeostasis (3,4,5,6,27).
The present study demonstrates that cell-specific loss of Stat3 in Agrp/Npy neurons results in a modest obesity phenotype accompanied by hyperphagia, hyperleptinemia, leptin hyposensitivity, and elevated Npy mRNA despite normal levels of Agrp mRNA.
Supplementary Material
Acknowledgments
We thank Charlotte Lee and Nina Balthsar for the Egfp immunostaining.
Footnotes
This work was supported by Research Grant 7-05-RA-84 from the American Diabetes Association (to R.G.M.) and Grant MLSC-27 from the Michigan Life Sciences Corridor. M.J.L. and M.R. received support from National Institutes of Health Grant DK068400. Body composition and energy expenditure measurements were performed at the Mouse Metabolic Phenotyping Center at the University of Washington (DK076126-01).
Disclosure Summary: Oregon Health and Science University (OHSU) and M.J.L. have a significant financial interest in Orexigen Therapeutics, Inc., a company that has licensed the POMC-EGFP transgenic mice and that may have a commercial interest in the results of this research and technology. This potential conflict of interest has been reviewed and managed by the OHSU Conflict of Interest in Research Committee and the Integrity Program Oversight Council. L.G., F.Y., K.H., H.H.H., G.J.M., K.T, S.A., M.R., and R.G.M. have nothing to disclose.
First Published Online April 10, 2008
Abbreviations: Agrp, Agouti-related protein; CFLIR, c-fos like immunoreactivity; CT, threshold cycle; ΔCT, Δ cycle threshold; Egfp, enhanced green fluorescent protein; FI, fluorescence intensity; HFD, high-fat diet; Lepr, leptin receptor; MCR, melanocortin receptor; Npy, neuropeptide Y; Pomc, proopiomelanocortin; QRT-PCR, quantitative RT-PCR; Socs, suppressor of cytokine signaling; Stat, signal transducer and activator of transcription; VO2, oxygen consumption.
References
- Friedman JM, Halaas JL 1998 Leptin and the regulation of body weight in mammals. Nature 395:763–770 [DOI] [PubMed] [Google Scholar]
- Cohen P, Zhao C, Cai X, Montez JM, Rohani SC, Feinstein P, Mombaerts P, Friedman JM 2001 Selective deletion of leptin receptor in neurons leads to obesity. J Clin Invest 108:1113–1121 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dhillon H, Zigman JM, Ye C, Lee CE, McGovern RA, Tang V, Kenny CD, Christiansen LM, White RD, Edelstein EA, Coppari R, Balthasar N, Cowley MA, Chua Jr S, Elmquist JK, Lowell BB 2006 Leptin directly activates SF1 neurons in the VMH, and this action by leptin is required for normal body-weight homeostasis. Neuron 49:191–203 [DOI] [PubMed] [Google Scholar]
- Grill HJ 2006 Distributed neural control of energy balance: contributions from hindbrain and hypothalamus. Obesity (Silver Spring) 14(Suppl 5):216S–221S [DOI] [PubMed] [Google Scholar]
- Leinninger GM, Myers Jr MG 2008 LRb signals act within a distributed network of leptin-responsive neurones to mediate leptin action. Acta Physiol (Oxf) 192:49–59 [DOI] [PubMed] [Google Scholar]
- Morton GJ, Cummings DE, Baskin DG, Barsh GS, Schwartz MW 2006 Central nervous system control of food intake and body weight. Nature 443:289–295 [DOI] [PubMed] [Google Scholar]
- Tartaglia LA, Dembski M, Weng X, Deng N, Culpepper J, Devos R, Richards GJ, Campfield LA, Clark FT, Deeds J, Muir C, Sanker S, Moriarty A, Moore KJ, Smutko JS, Mays GG, Wool EA, Monroe CA, Tepper RI 1995 Identification and expression cloning of a leptin receptor, OB-R. Cell 83:1263–1271 [DOI] [PubMed] [Google Scholar]
- Gao Q, Wolfgang MJ, Neschen S, Morino K, Horvath TL, Shulman GI, Fu XY 2004 Disruption of neural signal transducer and activator of transcription 3 causes obesity, diabetes, infertility, and thermal dysregulation. Proc Natl Acad Sci USA 101:4661–4666 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bates SH, Stearns WH, Dundon TA, Schubert M, Tso AW, Wang Y, Banks AS, Lavery HJ, Haq AK, Maratos-Flier E, Neel BG, Schwartz MW, Myers Jr MG 2003 STAT3 signalling is required for leptin regulation of energy balance but not reproduction. Nature 421:856–859 [DOI] [PubMed] [Google Scholar]
- Cowley MA, Smart JL, Rubinstein M, Cerdan MG, Diano S, Horvath TL, Cone RD, Low MJ 2001 Leptin activates anorexigenic POMC neurons through a neural network in the arcuate nucleus. Nature 411:480–484 [DOI] [PubMed] [Google Scholar]
- Schwartz MW, Seeley RJ, Woods SC, Weigle DS, Campfield LA, Burn P, Baskin DG 1997 Leptin increases hypothalamic pro-opiomelanocortin mRNA expression in the rostral arcuate nucleus. Diabetes 46:2119–2123 [DOI] [PubMed] [Google Scholar]
- Takahashi KA, Cone RD 2005 Fasting induces a large, leptin-dependent increase in the intrinsic action potential frequency of orexigenic arcuate nucleus neuropeptide Y/agouti-related protein neurons. Endocrinology 146:1043–1047 [DOI] [PubMed] [Google Scholar]
- Stanley BG, Kyrkouli SE, Lampert S, Leibowitz SF 1986 Neuropeptide Y chronically injected into the hypothalamus: a powerful neurochemical inducer of hyperphagia and obesity. Peptides 7:1189–1192 [DOI] [PubMed] [Google Scholar]
- Taylor K, Lester E, Hudson B, Ritter S 2007 Hypothalamic and hindbrain NPY, AGRP and NE increase consummatory feeding responses. Physiol Behav 90:744–750 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Balthasar N, Coppari R, McMinn J, Liu SM, Lee CE, Tang V, Kenny CD, McGovern RA, Chua Jr SC, Elmquist JK, Lowell BB 2004 Leptin receptor signaling in POMC neurons is required for normal body weight homeostasis. Neuron 42:983–991 [DOI] [PubMed] [Google Scholar]
- Xu AW, Ste. Marie L, Kaelin CB, Barsh GS 2007 Inactivation of signal transducer and activator of transcription 3 in proopiomelanocortin (Pomc) neurons causes decreased pomc expression, mild obesity, and defects in compensatory refeeding. Endocrinology 148:72–80 [DOI] [PubMed] [Google Scholar]
- Kaelin CB, Gong L, Xu AW, Yao F, Hockman K, Morton GJ, Schwartz MW, Barsh GS, MacKenzie RG 2006 Signal transducer and activator of transcription (stat) binding sites but not stat3 are required for fasting-induced transcription of agouti-related protein messenger ribonucleic acid. Mol Endocrinol 20:2591–2602 [DOI] [PubMed] [Google Scholar]
- Fink L, Seeger W, Ermert L, Hanze J, Stahl U, Grimminger F, Kummer W, Bohle RM 1998 Real-time quantitative RT-PCR after laser-assisted cell picking. Nat Med 4:1329–1333 [DOI] [PubMed] [Google Scholar]
- Livak KJ, Schmittgen TD 2001 Analysis of relative gene expression data using real-time quantitative PCR and the 2[-ΔΔC(T)] method. Methods 25:402–408 [DOI] [PubMed] [Google Scholar]
- Cui Y, Huang L, Elefteriou F, Yang G, Shelton JM, Giles JE, Oz OK, Pourbahrami T, Lu CY, Richardson JA, Karsenty G, Li C 2004 Essential role of STAT3 in body weight and glucose homeostasis. Mol Cell Biol 24:258–269 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maffei M, Halaas J, Ravussin E, Pratley RE, Lee GH, Zhang Y, Fei H, Kim S, Lallone R, Ranganathan S, Kern PA, Friedman JM 1995 Leptin levels in human and rodent: measurement of plasma leptin and ob RNA in obese and weight-reduced subjects. Nat Med 1:1155–1161 [DOI] [PubMed] [Google Scholar]
- Piper ML, Unger EK, Myers Jr MG, Xu AW 2007 Specific physiological roles for Stat3 in leptin receptor-expressing neurons. Mol Endocrinol 22:751–759 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bates SH, Kulkarni RN, Seifert M, Myers Jr MG 2005 Roles for leptin receptor/STAT3-dependent and -independent signals in the regulation of glucose homeostasis. Cell Metab 1:169–178 [DOI] [PubMed] [Google Scholar]
- Enriori PJ, Evans AE, Sinnayah P, Cowley MA 2006 Leptin resistance and obesity. Obesity (Silver Spring) 14(Suppl 5):254S–258S [DOI] [PubMed] [Google Scholar]
- Munzberg H, Myers Jr MG 2005 Molecular and anatomical determinants of central leptin resistance. Nat Neurosci 8:566–570 [DOI] [PubMed] [Google Scholar]
- Munzberg H, Flier JS, Bjorbaek C 2004 Region-specific leptin resistance within the hypothalamus of diet-induced obese mice. Endocrinology 145:4880–4889 [DOI] [PubMed] [Google Scholar]
- Balthasar N 2006 Genetic dissection of neuronal pathways controlling energy homeostasis. Obesity (Silver Spring) 14(Suppl 5):222S–227S [DOI] [PubMed] [Google Scholar]
- Hommel JD, Trinko R, Sears RM, Georgescu D, Liu ZW, Gao XB, Thurmon JJ, Marinelli M, DiLeone RJ 2006 Leptin receptor signaling in midbrain dopamine neurons regulates feeding. Neuron 51:801–810 [DOI] [PubMed] [Google Scholar]
- van den Top M, Lee K, Whyment AD, Blanks AM, Spanswick D 2004 Orexigen-sensitive NPY/AgRP pacemaker neurons in the hypothalamic arcuate nucleus. Nat Neurosci 7:493–494 [DOI] [PubMed] [Google Scholar]
- Munzberg H, Jobst EE, Bates SH, Jones J, Villanueva E, Leshan R, Bjornholm M, Elmquist J, Sleeman M, Cowley MA, Myers Jr MG 2007 Appropriate inhibition of orexigenic hypothalamic arcuate nucleus neurons independently of leptin receptor/STAT3 signaling. J Neurosci 27:69–74 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Claret M, Smith MA, Batterham RL, Selman C, Choudhury AI, Fryer LG, Clements M, Al-Qassab H, Heffron H, Xu AW, Speakman JR, Barsh GS, Viollet B, Vaulont S, Ashford ML, Carling D, Withers DJ 2007 AMPK is essential for energy homeostasis regulation and glucose sensing by POMC and AgRP neurons. J Clin Invest 117:2325–2336 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coppola A, Liu ZW, Andrews ZB, Paradis E, Roy MC, Friedman JM, Ricquier D, Richard D, Horvath TL, Gao XB, Diano S 2007 A central thermogenic-like mechanism in feeding regulation: an interplay between arcuate nucleus T3 and UCP2. Cell Metab 5:21–33 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Konner AC, Janoschek R, Plum L, Jordan SD, Rother E, Ma X, Xu C, Enriori P, Hampel B, Barsh GS, Kahn CR, Cowley MA, Ashcroft FM, Bruning JC 2007 Insulin action in AgRP-expressing neurons is required for suppression of hepatic glucose production. Cell Metab 5:438–449 [DOI] [PubMed] [Google Scholar]
- Nakazato M, Murakami N, Date Y, Kojima M, Matsuo H, Kangawa K, Matsukura S 2001 A role for ghrelin in the central regulation of feeding. Nature 409:194–198 [DOI] [PubMed] [Google Scholar]
- Xu AW, Kaelin CB, Takeda K, Akira S, Schwartz MW, Barsh GS 2005 PI3K integrates the action of insulin and leptin on hypothalamic neurons. J Clin Invest 115:951–958 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kitamura T, Feng Y, Kitamura YI, Chua Jr SC, Xu AW, Barsh GS, Rossetti L, Accili D 2006 Forkhead protein FoxO1 mediates Agrp-dependent effects of leptin on food intake. Nat Med 12:534–540 [DOI] [PubMed] [Google Scholar]
- Mutze J, Roth J, Gerstberger R, Hubschle T 2007 Nuclear translocation of the transcription factor STAT5 in the rat brain after systemic leptin administration. Neurosci Lett 417:286–291 [DOI] [PubMed] [Google Scholar]
- Morrison CD, Morton GJ, Niswender KD, Gelling RW, Schwartz MW 2005 Leptin inhibits hypothalamic Npy and Agrp gene expression via a mechanism that requires phosphatidylinositol 3-OH-kinase signaling. Am J Physiol Endocrinol Metab 289:E1051–E1057 [DOI] [PubMed] [Google Scholar]
- Sakkou M, Wiedmer P, Anlag K, Hamm A, Seuntjens E, Ettwiller L, Tschop MH, Treier M 2007 A role for brain-specific homeobox factor bsx in the control of hyperphagia and locomotory behavior. Cell Metab 5:450–463 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.