Abstract
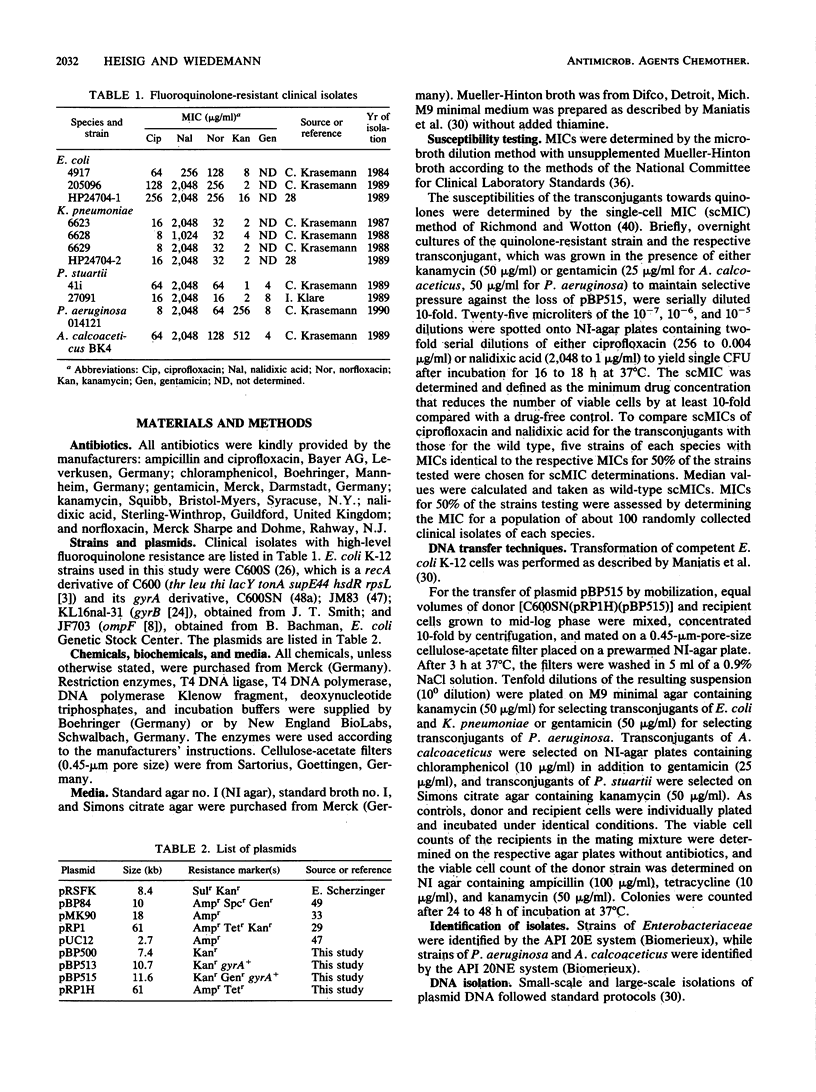
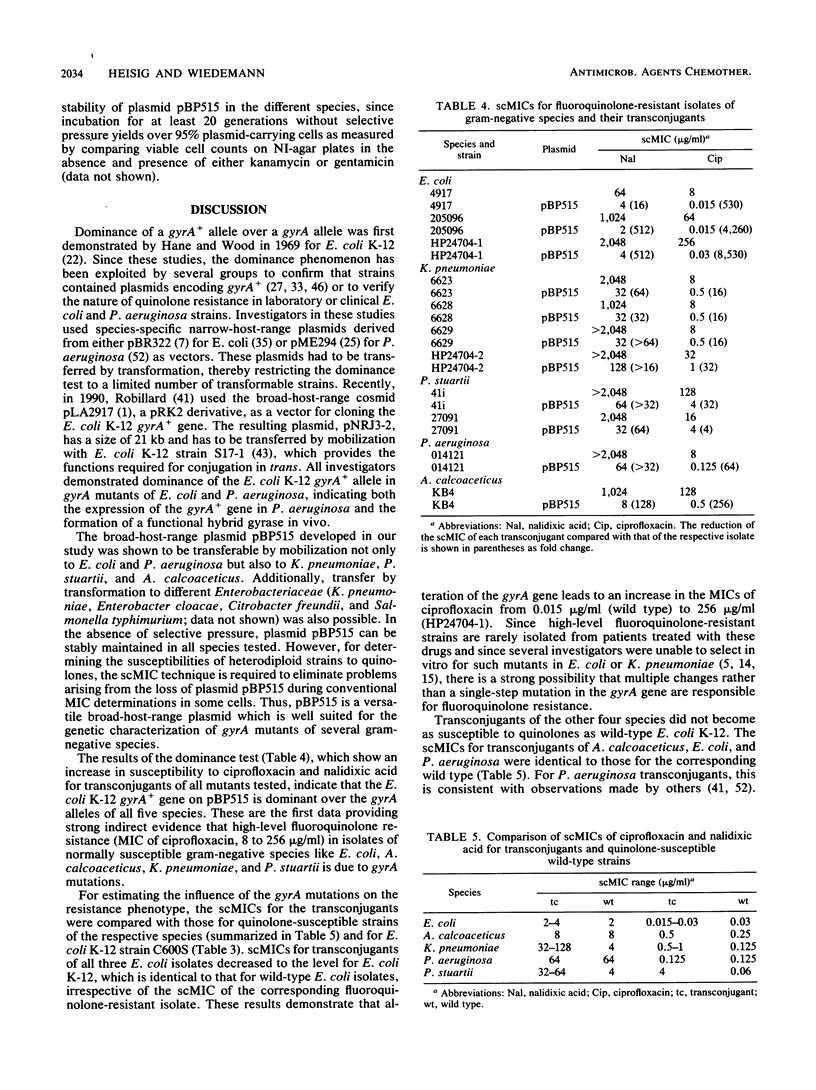
The gyrA genotypes of ciprofloxacin-resistant clinical isolates of Escherichia coli (n = 3), Klebsiella pneumoniae (n = 4), Providencia stuartii (n = 2), Pseudomonas aeruginosa (n = 1), and Acinetobacter calcoaceticus (n = 1) were analyzed in a dominance test. This test is based on the dominance of a wild-type gyrA gene (gyrA+) over the quinolone resistance allele (gyrA) in a heterodiploid strain. Plasmid pBP515, developed to carry the gyrA+ gene of E. coli K-12 on a broad-host-range vector derived from pRSF1010, was used to obtain heterodiploid strains. Plasmid pBP515 encodes kanamycin and gentamicin resistance and is transferable via mobilization by a pRP1-derived helper plasmid (pRP1H) to strains of several gram-negative species. After the introduction of pBP515, single-cell MICs (as measured by reduction of the viable cell count) of ciprofloxacin and nalidixic acid decreased by 4- to greater than 8,000-fold for all strains tested, and 8 of the 11 strains regained ciprofloxacin susceptibilities similar to those of the respective wild types. The results indicate that (i) high-level fluoroquinolone resistance in clinical isolates of E. coli, K. pneumoniae, P. aeruginosa, and A. calcoaceticus can result from mutational alteration of the gyrA gene, and (ii) gyrA mutations are involved in high levels of fluoroquinolone resistance in P. stuartii. Additional mutations outside the gyrA locus may contribute to resistance in K. pneumoniae and P. stuartii.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Allen L. N., Hanson R. S. Construction of broad-host-range cosmid cloning vectors: identification of genes necessary for growth of Methylobacterium organophilum on methanol. J Bacteriol. 1985 Mar;161(3):955–962. doi: 10.1128/jb.161.3.955-962.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aoyama H., Sato K., Kato T., Hirai K., Mitsuhashi S. Norfloxacin resistance in a clinical isolate of Escherichia coli. Antimicrob Agents Chemother. 1987 Oct;31(10):1640–1641. doi: 10.1128/aac.31.10.1640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Appleyard R K. Segregation of Lambda Lysogenicity during Bacterial Recombination in Escherichia Coli K12. Genetics. 1954 Jul;39(4):429–439. doi: 10.1093/genetics/39.4.429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bagdasarian M., Lurz R., Rückert B., Franklin F. C., Bagdasarian M. M., Frey J., Timmis K. N. Specific-purpose plasmid cloning vectors. II. Broad host range, high copy number, RSF1010-derived vectors, and a host-vector system for gene cloning in Pseudomonas. Gene. 1981 Dec;16(1-3):237–247. doi: 10.1016/0378-1119(81)90080-9. [DOI] [PubMed] [Google Scholar]
- Barry A. L., Jones R. N. Cross-resistance among cinoxacin, ciprofloxacin, DJ-6783, enoxacin, nalidixic acid, norfloxacin, and oxolinic acid after in vitro selection of resistant populations. Antimicrob Agents Chemother. 1984 Jun;25(6):775–777. doi: 10.1128/aac.25.6.775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bolivar F., Rodriguez R. L., Greene P. J., Betlach M. C., Heyneker H. L., Boyer H. W., Crosa J. H., Falkow S. Construction and characterization of new cloning vehicles. II. A multipurpose cloning system. Gene. 1977;2(2):95–113. [PubMed] [Google Scholar]
- Chai T. J., Foulds J. Escherichia coli K-12 tolF mutants: alterations in protein composition of the outer membrane. J Bacteriol. 1977 May;130(2):781–786. doi: 10.1128/jb.130.2.781-786.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chamberland S., Malouin F., Rabin H. R., Schollaardt T., Parr T. R., Jr, Bryan L. E. Persistence of Pseudomonas aeruginosa during ciprofloxacin therapy of a cystic fibrosis patient: transient resistance to quinolones and protein F-deficiency. J Antimicrob Chemother. 1990 Jun;25(6):995–1010. doi: 10.1093/jac/25.6.995. [DOI] [PubMed] [Google Scholar]
- Cheng A. F., Li M. K., Ling T. K., French G. L. Emergence of ofloxacin-resistant Citrobacter freundii and Pseudomonas maltophilia after ofloxacin therapy. J Antimicrob Chemother. 1987 Aug;20(2):283–285. doi: 10.1093/jac/20.2.283-a. [DOI] [PubMed] [Google Scholar]
- Chin N. X., Figueredo V. M., Novelli A., Neu H. C. In vitro activity of temafloxacin, a new difluoro quinolone antimicrobial agent. Eur J Clin Microbiol Infect Dis. 1988 Feb;7(1):58–63. doi: 10.1007/BF01962176. [DOI] [PubMed] [Google Scholar]
- Cullen M. E., Wyke A. W., Kuroda R., Fisher L. M. Cloning and characterization of a DNA gyrase A gene from Escherichia coli that confers clinical resistance to 4-quinolones. Antimicrob Agents Chemother. 1989 Jun;33(6):886–894. doi: 10.1128/aac.33.6.886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Datta N., Hedges R. W. Host ranges of R factors. J Gen Microbiol. 1972 May;70(3):453–460. doi: 10.1099/00221287-70-3-453. [DOI] [PubMed] [Google Scholar]
- Duckworth G. J., Williams J. D. Frequency of appearance of resistant variants to norfloxacin and nalidixic acid. J Antimicrob Chemother. 1984 May;13 (Suppl B):33–38. doi: 10.1093/jac/13.suppl_b.33. [DOI] [PubMed] [Google Scholar]
- Felmingham D., Foxall P., O'Hare M. D., Webb G., Ghosh G., Grüneberg R. N. Resistance studies with ofloxacin. J Antimicrob Chemother. 1988 Sep;22 (Suppl 100):27–34. doi: 10.1093/jac/22.supplement_c.27. [DOI] [PubMed] [Google Scholar]
- Fujimaki K., Fujii T., Aoyama H., Sato K., Inoue Y., Inoue M., Mitsuhashi S. Quinolone resistance in clinical isolates of Serratia marcescens. Antimicrob Agents Chemother. 1989 May;33(5):785–787. doi: 10.1128/aac.33.5.785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gellert M. DNA topoisomerases. Annu Rev Biochem. 1981;50:879–910. doi: 10.1146/annurev.bi.50.070181.004311. [DOI] [PubMed] [Google Scholar]
- Gellert M., Mizuuchi K., O'Dea M. H., Itoh T., Tomizawa J. I. Nalidixic acid resistance: a second genetic character involved in DNA gyrase activity. Proc Natl Acad Sci U S A. 1977 Nov;74(11):4772–4776. doi: 10.1073/pnas.74.11.4772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gellert M., O'Dea M. H., Itoh T., Tomizawa J. Novobiocin and coumermycin inhibit DNA supercoiling catalyzed by DNA gyrase. Proc Natl Acad Sci U S A. 1976 Dec;73(12):4474–4478. doi: 10.1073/pnas.73.12.4474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giamarellou H., Galanakis N., Dendrinos C., Stefanou J., Daphnis E., Daikos G. K. Evaluation of ciprofloxacin in the treatment of Pseudomonas aeruginosa infections. Eur J Clin Microbiol. 1986 Apr;5(2):232–235. doi: 10.1007/BF02013996. [DOI] [PubMed] [Google Scholar]
- Guerry P., van Embden J., Falkow S. Molecular nature of two nonconjugative plasmids carrying drug resistance genes. J Bacteriol. 1974 Feb;117(2):619–630. doi: 10.1128/jb.117.2.619-630.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hane M. W., Wood T. H. Escherichia coli K-12 mutants resistant to nalidixic acid: genetic mapping and dominance studies. J Bacteriol. 1969 Jul;99(1):238–241. doi: 10.1128/jb.99.1.238-241.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Higgins N. P., Peebles C. L., Sugino A., Cozzarelli N. R. Purification of subunits of Escherichia coli DNA gyrase and reconstitution of enzymatic activity. Proc Natl Acad Sci U S A. 1978 Apr;75(4):1773–1777. doi: 10.1073/pnas.75.4.1773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Inoue S., Ohue T., Yamagishi J., Nakamura S., Shimizu M. Mode of incomplete cross-resistance among pipemidic, piromidic, and nalidixic acids. Antimicrob Agents Chemother. 1978 Aug;14(2):240–245. doi: 10.1128/aac.14.2.240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Itoh Y., Soldati L., Leisinger T., Haas D. Low- and intermediate-copy-number cloning vectors based on the Pseudomonas plasmid pVS1. Antonie Van Leeuwenhoek. 1988;54(6):567–573. doi: 10.1007/BF00588392. [DOI] [PubMed] [Google Scholar]
- Kratz J., Schmidt F., Wiedemann B. Characterization of Tn2411 and Tn2410, two transposons derived from R-plasmid R1767 and related to Tn2603 and Tn21. J Bacteriol. 1983 Sep;155(3):1333–1342. doi: 10.1128/jb.155.3.1333-1342.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lampe M. F., Bott K. F. Genetic and physical organization of the cloned gyrA and gyrB genes of Bacillus subtilis. J Bacteriol. 1985 Apr;162(1):78–84. doi: 10.1128/jb.162.1.78-84.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- López-Brea M., Alarcón T. Isolation of fluoroquinolone-resistant Escherichia coli and Klebsiella pneumoniae from an infected Hickman catheter. Eur J Clin Microbiol Infect Dis. 1990 May;9(5):345–347. doi: 10.1007/BF01973741. [DOI] [PubMed] [Google Scholar]
- Masecar B. L., Celesk R. A., Robillard N. J. Analysis of acquired ciprofloxacin resistance in a clinical strain of Pseudomonas aeruginosa. Antimicrob Agents Chemother. 1990 Feb;34(2):281–286. doi: 10.1128/aac.34.2.281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mehtar S., Drabu Y., Blakemore P. Ciprofloxacin in the treatment of infections caused by gentamicin-resistant gram-negative bacteria. Eur J Clin Microbiol. 1986 Apr;5(2):248–251. doi: 10.1007/BF02014001. [DOI] [PubMed] [Google Scholar]
- Mizuuchi K., Mizuuchi M., O'Dea M. H., Gellert M. Cloning and simplified purification of Escherichia coli DNA gyrase A and B proteins. J Biol Chem. 1984 Jul 25;259(14):9199–9201. [PubMed] [Google Scholar]
- Mizuuchi K., O'Dea M. H., Gellert M. DNA gyrase: subunit structure and ATPase activity of the purified enzyme. Proc Natl Acad Sci U S A. 1978 Dec;75(12):5960–5963. doi: 10.1073/pnas.75.12.5960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakamura S., Nakamura M., Kojima T., Yoshida H. gyrA and gyrB mutations in quinolone-resistant strains of Escherichia coli. Antimicrob Agents Chemother. 1989 Feb;33(2):254–255. doi: 10.1128/aac.33.2.254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oram M., Fisher L. M. 4-Quinolone resistance mutations in the DNA gyrase of Escherichia coli clinical isolates identified by using the polymerase chain reaction. Antimicrob Agents Chemother. 1991 Feb;35(2):387–389. doi: 10.1128/aac.35.2.387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paganoni R., Herzog C., Braunsteiner A., Hohl P. Fleroxacin: in-vitro activity worldwide against 20,807 clinical isolates and comparison to ciprofloxacin and norfloxacin. J Antimicrob Chemother. 1988 Oct;22 (Suppl 500):3–17. doi: 10.1093/jac/22.supplement_d.3. [DOI] [PubMed] [Google Scholar]
- Piddock L. J., Wise R. Mechanisms of resistance to quinolones and clinical perspectives. J Antimicrob Chemother. 1989 Apr;23(4):475–480. doi: 10.1093/jac/23.4.475. [DOI] [PubMed] [Google Scholar]
- Richmond M. H., Wotton S. Comparative study of seven cephalosporins: susceptibility to beta-lactamases and ability to penetrate the surface layers of Escherichia coli. Antimicrob Agents Chemother. 1976 Aug;10(2):219–222. doi: 10.1128/aac.10.2.219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robillard N. J. Broad-host-range gyrase A gene probe. Antimicrob Agents Chemother. 1990 Oct;34(10):1889–1894. doi: 10.1128/aac.34.10.1889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanders C. C. Ciprofloxacin: in vitro activity, mechanism of action, and resistance. Rev Infect Dis. 1988 May-Jun;10(3):516–527. doi: 10.1093/clinids/10.3.516. [DOI] [PubMed] [Google Scholar]
- Sreedharan S., Oram M., Jensen B., Peterson L. R., Fisher L. M. DNA gyrase gyrA mutations in ciprofloxacin-resistant strains of Staphylococcus aureus: close similarity with quinolone resistance mutations in Escherichia coli. J Bacteriol. 1990 Dec;172(12):7260–7262. doi: 10.1128/jb.172.12.7260-7262.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sugino A., Peebles C. L., Kreuzer K. N., Cozzarelli N. R. Mechanism of action of nalidixic acid: purification of Escherichia coli nalA gene product and its relationship to DNA gyrase and a novel nicking-closing enzyme. Proc Natl Acad Sci U S A. 1977 Nov;74(11):4767–4771. doi: 10.1073/pnas.74.11.4767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swanberg S. L., Wang J. C. Cloning and sequencing of the Escherichia coli gyrA gene coding for the A subunit of DNA gyrase. J Mol Biol. 1987 Oct 20;197(4):729–736. doi: 10.1016/0022-2836(87)90479-7. [DOI] [PubMed] [Google Scholar]
- Vieira J., Messing J. The pUC plasmids, an M13mp7-derived system for insertion mutagenesis and sequencing with synthetic universal primers. Gene. 1982 Oct;19(3):259–268. doi: 10.1016/0378-1119(82)90015-4. [DOI] [PubMed] [Google Scholar]
- Wang J. C. DNA topoisomerases. Annu Rev Biochem. 1985;54:665–697. doi: 10.1146/annurev.bi.54.070185.003313. [DOI] [PubMed] [Google Scholar]
- Wiedemann B., Meyer J. F., Zühlsdorf M. T. Insertions of resistance genes into Tn21-like transposons. J Antimicrob Chemother. 1986 Oct;18 (Suppl 100):85–92. doi: 10.1093/jac/18.supplement_c.85. [DOI] [PubMed] [Google Scholar]
- Yamagishi J., Yoshida H., Yamayoshi M., Nakamura S. Nalidixic acid-resistant mutations of the gyrB gene of Escherichia coli. Mol Gen Genet. 1986 Sep;204(3):367–373. doi: 10.1007/BF00331012. [DOI] [PubMed] [Google Scholar]
- Yoshida H., Bogaki M., Nakamura M., Nakamura S. Quinolone resistance-determining region in the DNA gyrase gyrA gene of Escherichia coli. Antimicrob Agents Chemother. 1990 Jun;34(6):1271–1272. doi: 10.1128/aac.34.6.1271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoshida H., Nakamura M., Bogaki M., Nakamura S. Proportion of DNA gyrase mutants among quinolone-resistant strains of Pseudomonas aeruginosa. Antimicrob Agents Chemother. 1990 Jun;34(6):1273–1275. doi: 10.1128/aac.34.6.1273. [DOI] [PMC free article] [PubMed] [Google Scholar]


