Abstract
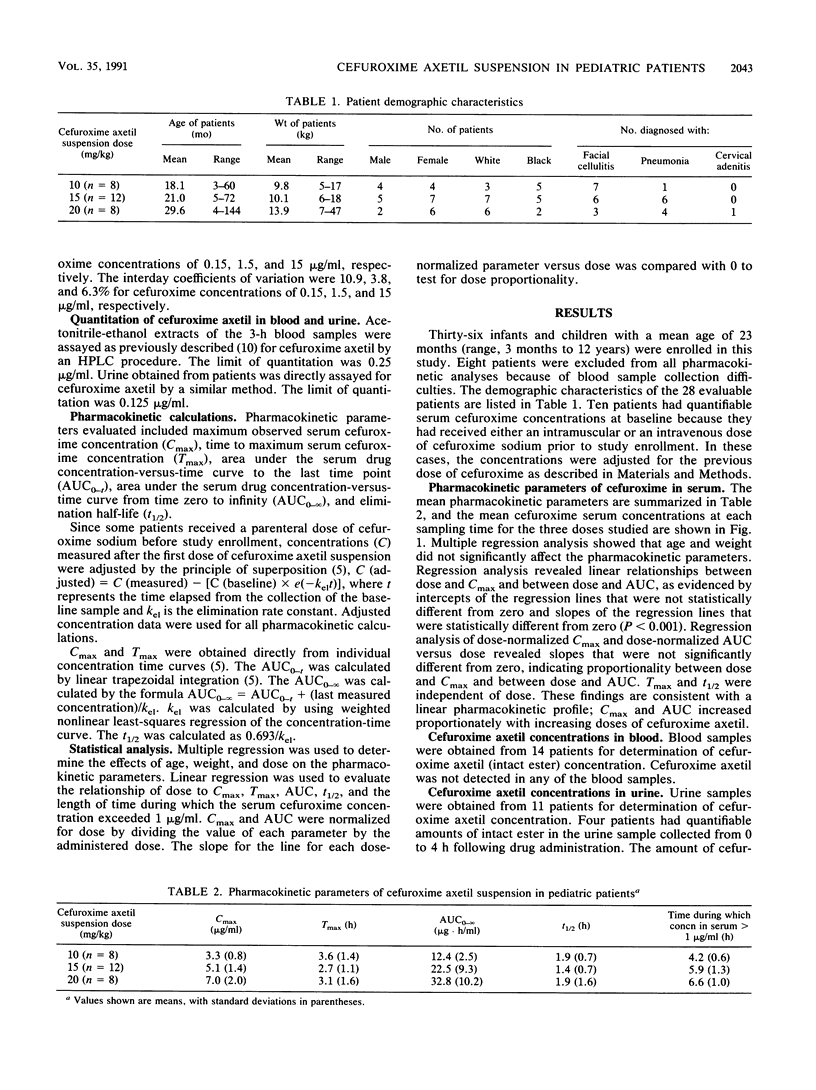
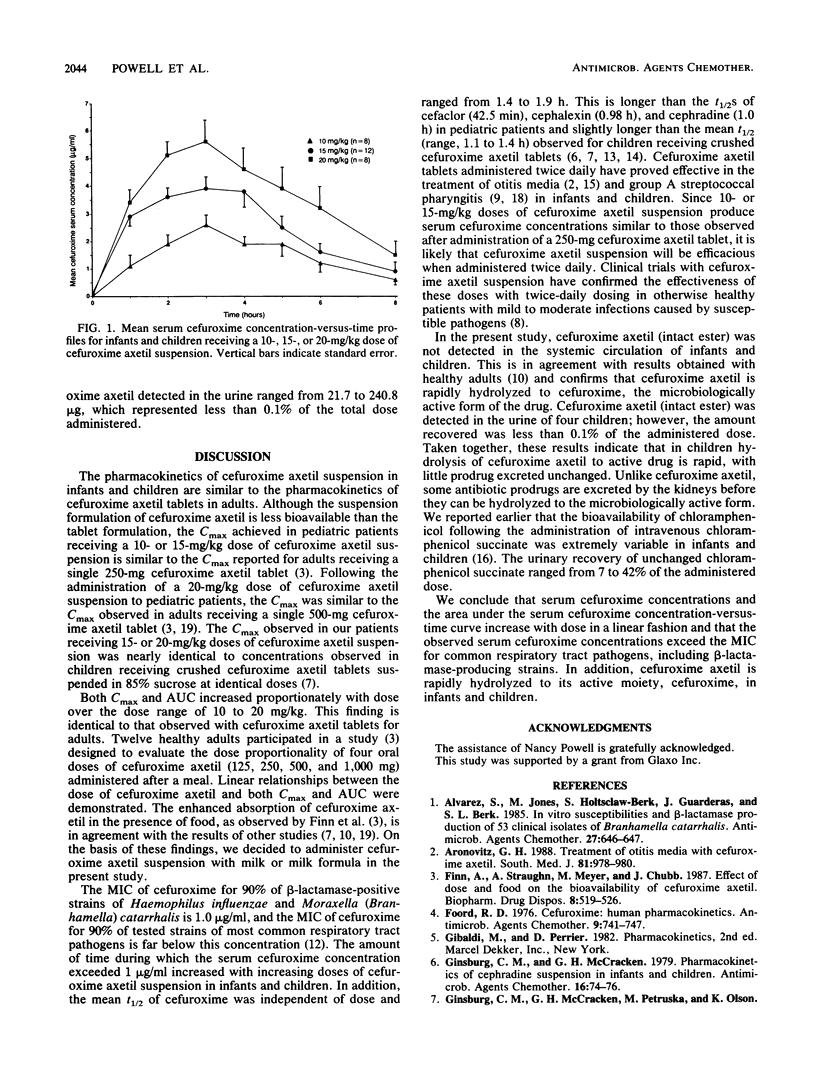
The pharmacokinetics of cefuroxime axetil suspension in 28 infants and children, ranging in age from 3 months to 12 years (mean, 23 months), were studied. Mean maximum serum cefuroxime concentrations of 3.3, 5.1, and 7.0 micrograms/ml were achieved 3.6, 2.7, and 3.1 h after the administration of doses of 10, 15, and 20 mg, respectively, of cefuroxime axetil suspension per kg of body weight together with milk or milk formula. These concentrations exceed the MICs for common respiratory tract pathogens, including beta-lactamase-producing strains of Haemophilus influenzae and Moraxella (Branhamella) catarrhalis. Following a 10- or 15-mg/kg dose, serum cefuroxime concentrations are similar to those achieved in adults following the administration of a 250-mg cefuroxime axetil tablet. There were linear relationships between dose and both maximum serum cefuroxime concentration and area under the serum drug concentration-verus-time curve. The mean half-life of cefuroxime in serum was independent of dose and ranged from 1.4 to 1.9 h. No cefuroxime axetil (intact ester) was detected in the blood. The intact ester in the urine of four children was measured; however, the amount recovered represented less than 0.1% of the administered dose.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Alvarez S., Jones M., Holtsclaw-Berk S., Guarderas J., Berk S. L. In vitro susceptibilities and beta-lactamase production of 53 clinical isolates of Branhamella catarrhalis. Antimicrob Agents Chemother. 1985 Apr;27(4):646–647. doi: 10.1128/aac.27.4.646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aronovitz G. H. Treatment of otitis media with cefuroxime axetil. South Med J. 1988 Aug;81(8):978–980. doi: 10.1097/00007611-198808000-00009. [DOI] [PubMed] [Google Scholar]
- Finn A., Straughn A., Meyer M., Chubb J. Effect of dose and food on the bioavailability of cefuroxime axetil. Biopharm Drug Dispos. 1987 Nov-Dec;8(6):519–526. doi: 10.1002/bdd.2510080604. [DOI] [PubMed] [Google Scholar]
- Foord R. D. Cefuroxime: human pharmacokinetics.. Antimicrob Agents Chemother. 1976 May;9(5):741–747. doi: 10.1128/aac.9.5.741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ginsburg C. M., McCracken G. H., Jr Pharmacokinetics of cephradine suspension infants and children. Antimicrob Agents Chemother. 1979 Jul;16(1):74–76. doi: 10.1128/aac.16.1.74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gooch W. M., 3rd, Swenson E., Higbee M. D., Cocchetto D. M., Evans E. C. Cefuroxime axetil and penicillin V compared in the treatment of group A beta-hemolytic streptococcal pharyngitis. Clin Ther. 1987;9(6):670–677. [PubMed] [Google Scholar]
- Harding S. M., Williams P. E., Ayrton J. Pharmacology of Cefuroxime as the 1-acetoxyethyl ester in volunteers. Antimicrob Agents Chemother. 1984 Jan;25(1):78–82. doi: 10.1128/aac.25.1.78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones R. N., Fuchs P. C., Gavan T. L., Gerlach E. H., Barry A. L., Thornsberry C. Cefuroxime, a new parenteral cephalosporin: collaborative in vitro susceptibility comparison with cephalothin against 5,887 clinical bacterial isolates. Antimicrob Agents Chemother. 1977 Jul;12(1):47–50. doi: 10.1128/aac.12.1.47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jorgensen J. H., Doern G. V., Maher L. A., Howell A. W., Redding J. S. Antimicrobial resistance among respiratory isolates of Haemophilus influenzae, Moraxella catarrhalis, and Streptococcus pneumoniae in the United States. Antimicrob Agents Chemother. 1990 Nov;34(11):2075–2080. doi: 10.1128/aac.34.11.2075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lode H., Stahlmann R., Koeppe P. Comparative pharmacokinetics of cephalexin, cefaclor, cefadroxil, and CGP 9000. Antimicrob Agents Chemother. 1979 Jul;16(1):1–6. doi: 10.1128/aac.16.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCracken G. H., Jr, Ginsburg C. M., Clahsen J. C., Thomas M. L. Pharmacologic evaluation of orally administered antibiotics in infants and children: effect of feeding on bioavailability. Pediatrics. 1978 Nov;62(5):738–743. [PubMed] [Google Scholar]
- Nahata M. C., Powell D. A. Bioavailability and clearance of chloramphenicol after intravenous chloramphenicol succinate. Clin Pharmacol Ther. 1981 Sep;30(3):368–372. doi: 10.1038/clpt.1981.174. [DOI] [PubMed] [Google Scholar]
- Neu H. C., Fu K. P. Cefuroxime, a beta-lactamase-resistant cephalosporin with a broad spectrum of gram-positive and -negative activity. Antimicrob Agents Chemother. 1978 Apr;13(4):657–664. doi: 10.1128/aac.13.4.657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pichichero M. E., Disney F. A., Aronovitz G. H., Ginsburg C., Stillerman M. A multicenter, randomized, single-blind evaluation of cefuroxime axetil and phenoxymethyl penicillin in the treatment of streptococcal pharyngitis. Clin Pediatr (Phila) 1987 Sep;26(9):453–458. doi: 10.1177/000992288702600904. [DOI] [PubMed] [Google Scholar]
- Sommers D. K., Van Wyk M., Williams P. E., Harding S. M. Pharmacokinetics and tolerance of cefuroxime axetil in volunteers during repeated dosing. Antimicrob Agents Chemother. 1984 Mar;25(3):344–347. doi: 10.1128/aac.25.3.344. [DOI] [PMC free article] [PubMed] [Google Scholar]


