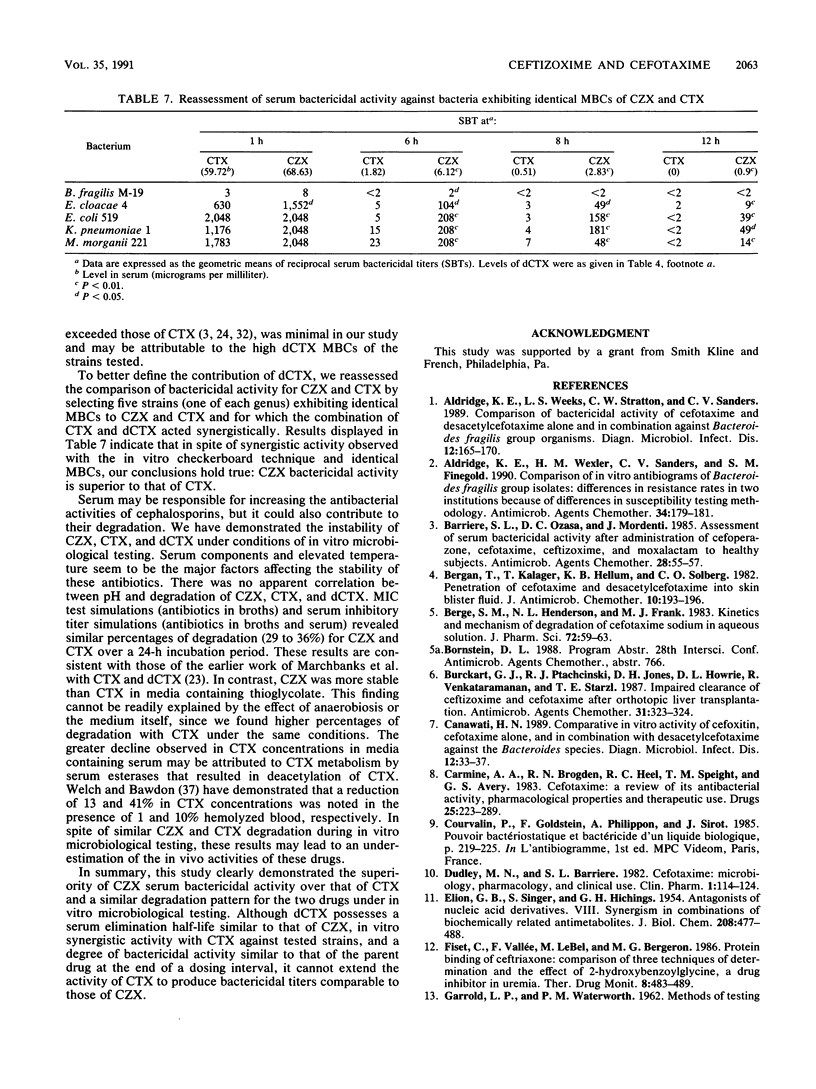
Abstract
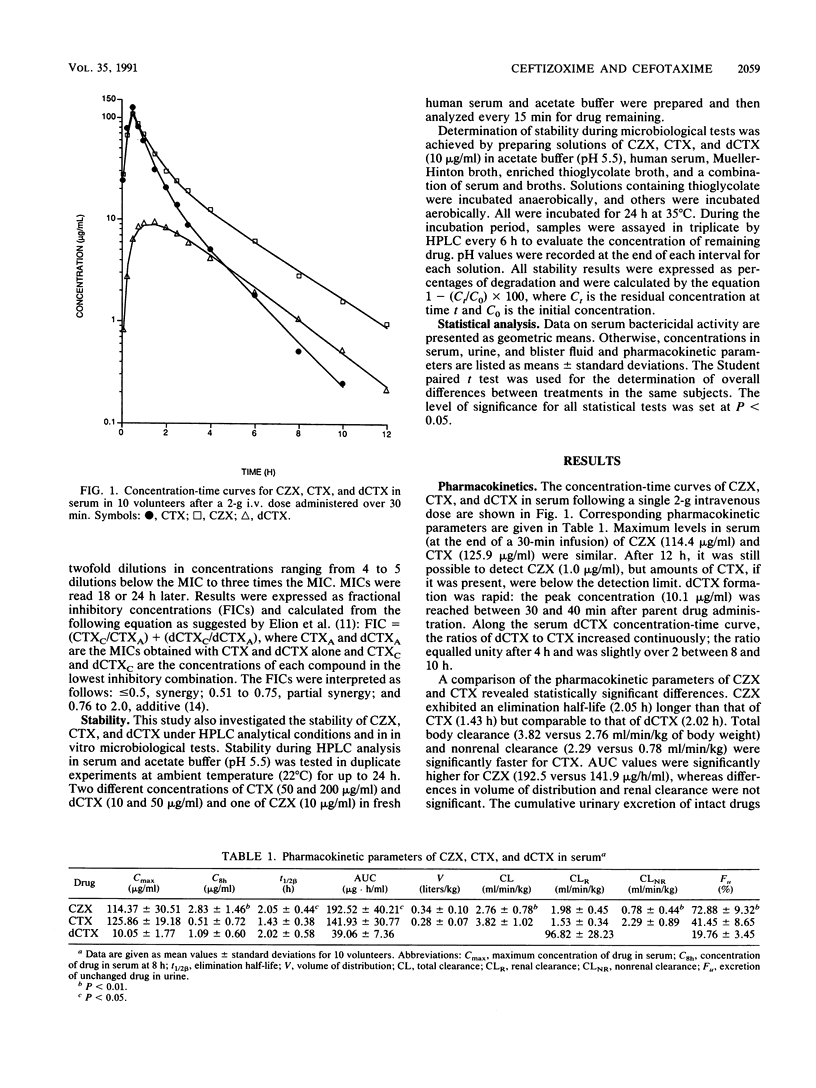
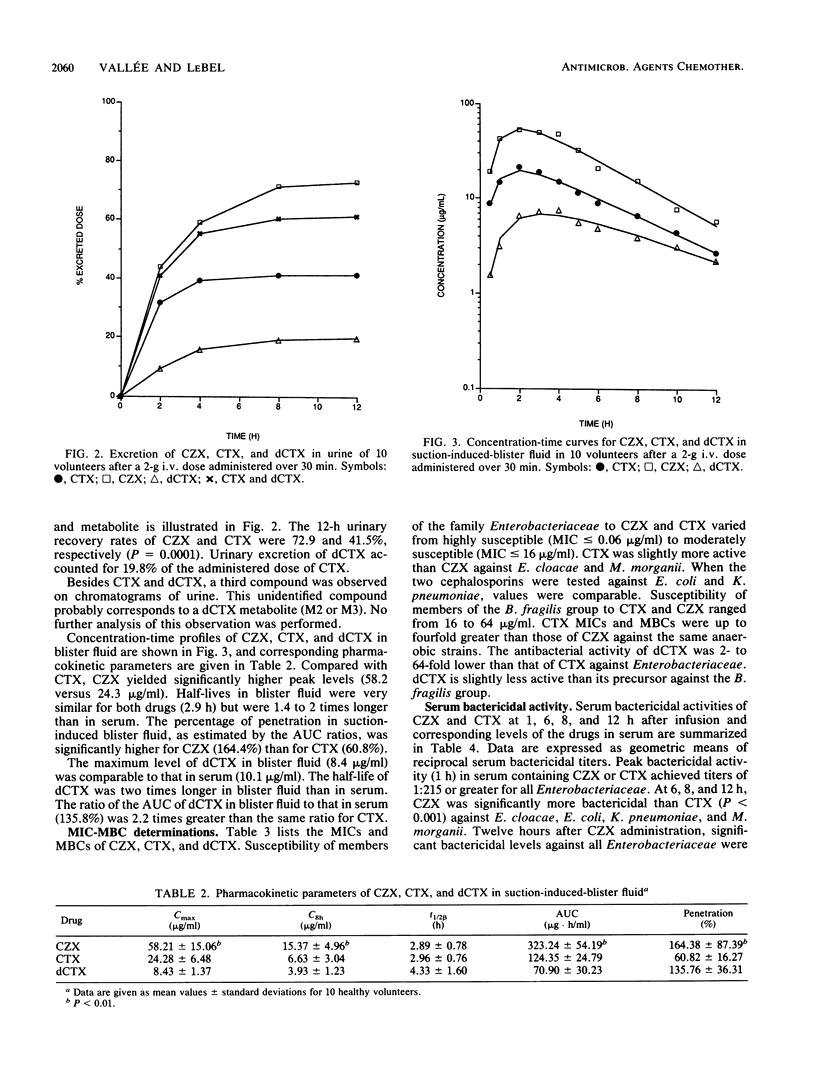
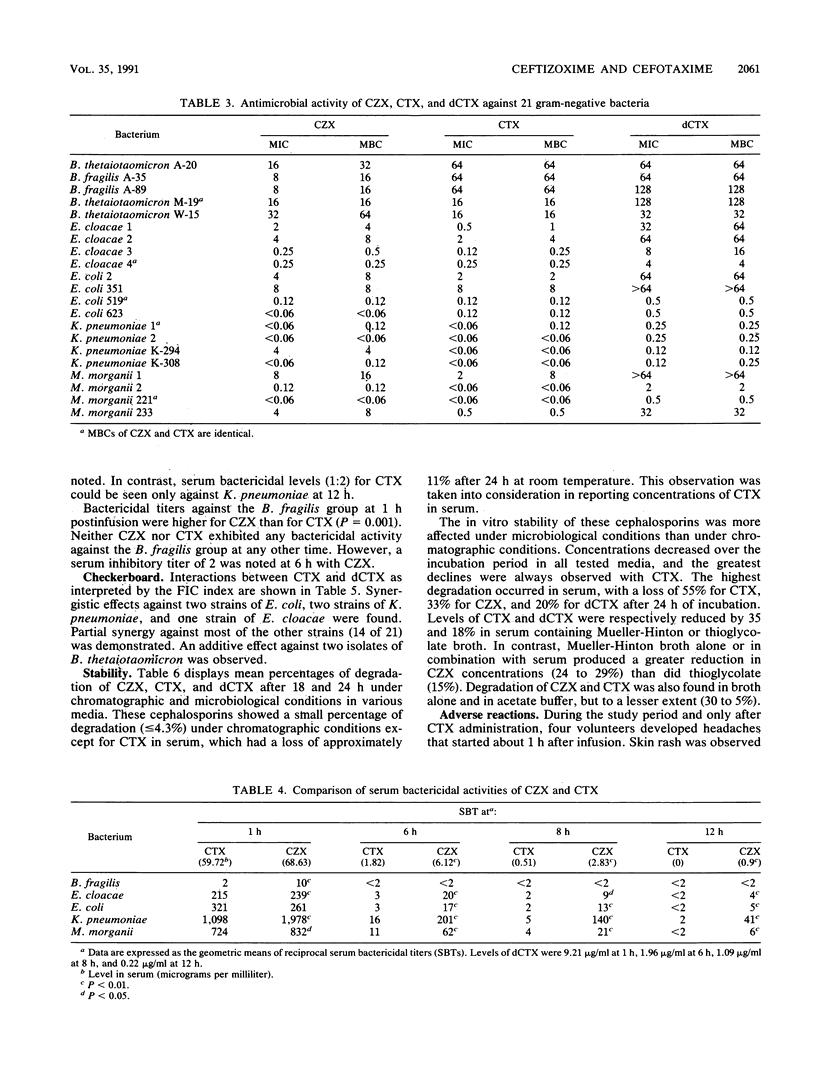
Single 2-g intravenous doses of ceftizoxime (CZX) and cefotaxime (CTX) were given over 30 min to 10 adult volunteers in a crossover manner on two separate occasions. Concentrations of CZX, CTX, and the primary metabolite of CTX, desacetylcefotaxime (dCTX), in serum, suction-induced-blister fluid, and urine were determined by high-pressure liquid chromatography. Pharmacokinetic parameters were estimated by using an extended least-squares modeling program (MKMODEL). CZX exhibited a half-life in serum (2.05 h) longer than that of CTX (1.43 h) but comparable to that of dCTX (2.02 h). The percentage of penetration in blister fluid, estimated by area under the curve ratios, was significantly higher for CZX (164.4%) than for CTX (60.8%). Serum bactericidal activity, determined for volunteer samples at 1, 6, 8, and 12 h after patients were dosed, against clinical isolates of the Bacteroides fragilis group, Enterobacter cloacae, Escherichia coli, Klebsiella pneumoniae, and Morganella morganii were significantly higher for CZX than those for CTX against members of the family Enterobacteriaceae at all times. Serum bactericidal titers against B. fragilis were also higher for CZX than for CTX at 1 h postinfusion. Neither CZX nor CTX exhibited any bactericidal activity at any other time against the B. fragilis group. In conclusion, the serum bactericidal activity of CZX was greater and more-prolonged than that of CTX against tested strains in spite of the in vitro synergistic contribution of dCTX to CTX, equal serum elimination half-lives of dCTX and CZX, and similar antibacterial activity and similar instability under microbiological testing for CZX and CTX.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aldridge K. E., Weeks L. S., Stratton C. W., Sanders C. V. Comparison of the bactericidal activity of cefotaxime and desacetylcefotaxime alone and in combination against Bacteroides fragilis group organisms. Diagn Microbiol Infect Dis. 1989 Mar-Apr;12(2):165–170. doi: 10.1016/0732-8893(89)90008-4. [DOI] [PubMed] [Google Scholar]
- Aldridge K. E., Wexler H. M., Sanders C. V., Finegold S. M. Comparison of in vitro antibiograms of Bacteroides fragilis group isolates: differences in resistance rates in two institutions because of differences in susceptibility testing methodology. Antimicrob Agents Chemother. 1990 Jan;34(1):179–181. doi: 10.1128/aac.34.1.179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barriere S. L., Ozasa D. C., Mordenti J. Assessment of serum bactericidal activity after administration of cefoperazone, cefotaxime, ceftizoxime, and moxalactam to healthy subjects. Antimicrob Agents Chemother. 1985 Jul;28(1):55–57. doi: 10.1128/aac.28.1.55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bergan T., Kalager T., Hellum K. B., Solberg C. O. Penetration of cefotaxime and desacetylcefotaxime into skin blister fluid. J Antimicrob Chemother. 1982 Sep;10(3):193–196. doi: 10.1093/jac/10.3.193. [DOI] [PubMed] [Google Scholar]
- Berge S. M., Henderson N. L., Frank M. J. Kinetics and mechanism of degradation of cefotaxime sodium in aqueous solution. J Pharm Sci. 1983 Jan;72(1):59–63. doi: 10.1002/jps.2600720114. [DOI] [PubMed] [Google Scholar]
- Burckart G. J., Ptachcinski R. J., Jones D. H., Howrie D. L., Venkataramanan R., Starzl T. E. Impaired clearance of ceftizoxime and cefotaxime after orthotopic liver transplantation. Antimicrob Agents Chemother. 1987 Feb;31(2):323–324. doi: 10.1128/aac.31.2.323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Canawati H. N. Comparative in vitro activity of cefoxitin, cefotaxime alone, and in combination with desacetylcefotaxime against the Bacteroides species. Diagn Microbiol Infect Dis. 1989 Jan-Feb;12(1):33–37. doi: 10.1016/0732-8893(89)90043-6. [DOI] [PubMed] [Google Scholar]
- Carmine A. A., Brogden R. N., Heel R. C., Speight T. M., Avery G. S. Cefotaxime. A review of its antibacterial activity, pharmacological properties and therapeutic use. Drugs. 1983 Mar;25(3):223–289. doi: 10.2165/00003495-198325030-00001. [DOI] [PubMed] [Google Scholar]
- Dudley M. N., Barriere S. L. Cefotaxime: microbiology, pharmacology, and clinical use. Clin Pharm. 1982 Mar-Apr;1(2):114–124. [PubMed] [Google Scholar]
- ELION G. B., SINGER S., HITCHINGS G. H. Antagonists of nucleic acid derivatives. VIII. Synergism in combinations of biochemically related antimetabolites. J Biol Chem. 1954 Jun;208(2):477–488. [PubMed] [Google Scholar]
- Fiset C., Vallée F., LeBel M., Bergeron M. G. Protein binding of ceftriaxone: comparison of three techniques of determination and the effect of 2-hydroxybenzoylglycine, a drug-binding inhibitor in uremia. Ther Drug Monit. 1986;8(4):483–489. [PubMed] [Google Scholar]
- Hall M. J., Middleton R. F., Westmacott D. The fractional inhibitory concentration (FIC) index as a measure of synergy. J Antimicrob Chemother. 1983 May;11(5):427–433. doi: 10.1093/jac/11.5.427. [DOI] [PubMed] [Google Scholar]
- Jenkins S. G. Activity of cefotaxime/desacetylcefotaxime with two aminoglycosides against gram-negative pathogens: an example of interactive synergy. Diagn Microbiol Infect Dis. 1989 Jan-Feb;12(1):51–55. doi: 10.1016/0732-8893(89)90046-1. [DOI] [PubMed] [Google Scholar]
- Korting H. C., Schäfer-Korting M., Haag R., Mutschler E. Plasma, cantharides blister fluid, and suction blister fluid levels of ceftizoxime after single intramuscular application for gonorrhea. Int J Clin Pharmacol Ther Toxicol. 1984 Apr;22(4):218–220. [PubMed] [Google Scholar]
- Limbert M., Seibert G., Schrinner E. The cooperation of cefotaxime and desacetyl-cefotaxime with respect to antibacterial activity and beta-lactamase stability. Infection. 1982;10(2):97–100. doi: 10.1007/BF01816732. [DOI] [PubMed] [Google Scholar]
- Lüthy R., Blaser J., Bonetti A., Simmen H., Wise R., Siegenthaler W. Comparative multiple-dose pharmacokinetics of cefotaxime, moxalactam, and ceftazidime. Antimicrob Agents Chemother. 1981 Nov;20(5):567–575. doi: 10.1128/aac.20.5.567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marchbanks C. R., Yost R. L., White R. L. Cefotaxime stability during in vitro microbiological testing. Antimicrob Agents Chemother. 1987 Sep;31(9):1375–1378. doi: 10.1128/aac.31.9.1375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moore E. S., Jimenez M., Barriere S. L., Cimino M. L., Fekety F. R. Additive and synergistic bactericidal activity contributed by desacetylcefotaxime during cefotaxime therapy. Clin Pharm. 1988 Dec;7(12):901–905. [PubMed] [Google Scholar]
- Neu H. C., Aswapokee N., Aswapokee P., Fu K. P. HR 756, a new cephalosporin active against gram-positive and gram-negative aerobic and anaerobic bacteria. Antimicrob Agents Chemother. 1979 Feb;15(2):273–281. doi: 10.1128/aac.15.2.273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neu H. C. Ceftizoxime: a beta-lactamase-stable, broad-spectrum cephalosporin. Pharmacokinetics, adverse effects and clinical use. Pharmacotherapy. 1984 Mar-Apr;4(2):47–60. doi: 10.1002/j.1875-9114.1984.tb03313.x. [DOI] [PubMed] [Google Scholar]
- Oizumi K., Hayashi I., Aonuma S., Konno K. In vitro activity of desacetylcefotaxime and the interaction with its parent compound, cefotaxime. Drugs. 1988;35 (Suppl 2):57–61. doi: 10.2165/00003495-198800352-00013. [DOI] [PubMed] [Google Scholar]
- Panneton A. C., Bergeron M. G., LeBel M. Pharmacokinetics and tissue penetration of fleroxacin after single and multiple 400- and 800-mg-dosage regimens. Antimicrob Agents Chemother. 1988 Oct;32(10):1515–1520. doi: 10.1128/aac.32.10.1515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quintiliani R., Nightingale C. H., Tilton R. Comparative pharmacokinetics of cefotaxime and ceftizoxime and the role of desacetylcefotaxime in the antibacterial activity of cefotaxime. Diagn Microbiol Infect Dis. 1984 Jun;2(3 Suppl):63S–70S. [PubMed] [Google Scholar]
- Reller L. B. Interaction of cefotaxime and desacetylcefotaxime against pathogenic bacteria. Assessment with the serum bactericidal test. Diagn Microbiol Infect Dis. 1984 Jun;2(3 Suppl):55S–61S. [PubMed] [Google Scholar]
- Richards D. M., Heel R. C. Ceftizoxime. A review of its antibacterial activity, pharmacokinetic properties and therapeutic use. Drugs. 1985 Apr;29(4):281–329. doi: 10.2165/00003495-198529040-00001. [DOI] [PubMed] [Google Scholar]
- Rosenblatt J. E. Antimicrobial susceptibility testing of anaerobic bacteria. Rev Infect Dis. 1984 Mar-Apr;6 (Suppl 1):S242–S248. doi: 10.1093/clinids/6.supplement_1.s242. [DOI] [PubMed] [Google Scholar]
- Shyu W. C., Quintiliani R., Nightingale C. H. An improved method to determine interstitial fluid pharmacokinetics. J Infect Dis. 1985 Dec;152(6):1328–1331. doi: 10.1093/infdis/152.6.1328. [DOI] [PubMed] [Google Scholar]
- Wasilauskas B. L. Effectiveness of cefotaxime alone and in combination with desacetylcefotaxime against Bacteroides fragilis. Diagn Microbiol Infect Dis. 1989 Jan-Feb;12(1):39–43. doi: 10.1016/0732-8893(89)90044-8. [DOI] [PubMed] [Google Scholar]
- Welch W. D., Bawdon R. E. Cefotaxime metabolism by hemolyzed blood: quantitation and inhibition of the deacetylation reaction. Diagn Microbiol Infect Dis. 1986 Feb;4(2):119–124. doi: 10.1016/0732-8893(86)90145-8. [DOI] [PubMed] [Google Scholar]


