Abstract
Routes of quinolone permeation in Pseudomonas aeruginosa were investigated by using sparfloxacin as a prototype compound. [14C]sparfloxacin cell labeling was 13 to 28% lower in three protein D2-deficient mutants resistant to imipenem than in their imipenem-susceptible counterparts. In four impermeability-type quinolone-resistant strains isolated from pefloxacin-treated animals, we observed two- to fourfold-greater resistance to imipenem, reduced protein D2 expression in the outer membrane according to Western blotting (immunoblotting), and 25 to 29% decreased cell labeling with imipenem. In a protein D2-producing strain but not in its protein D2-deficient isogenic mutant, uptake of [14C]sparfloxacin was strongly inhibited by L-lysine and imipenem, which act as substrates for protein D2. Conversely, binding of [14C]imipenem in a porin D2-positive strain was reduced by sparfloxacin but not by the nonamphoteric quinolone nalidixic acid. Sparfloxacin, imipenem, and lysine possess a carboxyl group and a potentially protonated nitrogen separated from each other by 0.64 to 1.07 nm as calculated by computer. Hence, protein D2 may catalyze facilitated diffusion for sparfloxacin, as it does for imipenem. In addition, pefloxacin-selected isolates contained 41 to 113% more 3-deoxy-D-mannooctulosonic acid than their quinolone-susceptible counterparts, with MIC increases of 2- to 4-fold for WIN-57273 (n-octanol-phosphate buffer partition coefficient, 13.139), 4- to 8-fold for difloxacin (partition coefficient, 3.093) and sparfloxacin (partition coefficient, 0.431), and 8- to 16-fold for norfloxacin (partition coefficient, 0.059) and ciprofloxacin (partition coefficient, 0.056). Thus, we hypothetize that in quinolone-selected strains, increased amounts of lipopolysaccharide form a permeability barrier that acts preferentially against hydrophilic quinolones.
Full text
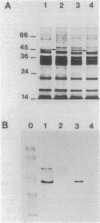
PDF





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Angus B. L., Carey A. M., Caron D. A., Kropinski A. M., Hancock R. E. Outer membrane permeability in Pseudomonas aeruginosa: comparison of a wild-type with an antibiotic-supersusceptible mutant. Antimicrob Agents Chemother. 1982 Feb;21(2):299–309. doi: 10.1128/aac.21.2.299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bedard J., Bryan L. E. Interaction of the fluoroquinolone antimicrobial agents ciprofloxacin and enoxacin with liposomes. Antimicrob Agents Chemother. 1989 Aug;33(8):1379–1382. doi: 10.1128/aac.33.8.1379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bryan L. E., O'Hara K., Wong S. Lipopolysaccharide changes in impermeability-type aminoglycoside resistance in Pseudomonas aeruginosa. Antimicrob Agents Chemother. 1984 Aug;26(2):250–255. doi: 10.1128/aac.26.2.250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chamberland S., Bayer A. S., Schollaardt T., Wong S. A., Bryan L. E. Characterization of mechanisms of quinolone resistance in Pseudomonas aeruginosa strains isolated in vitro and in vivo during experimental endocarditis. Antimicrob Agents Chemother. 1989 May;33(5):624–634. doi: 10.1128/aac.33.5.624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chamberland S., Malouin F., Rabin H. R., Schollaardt T., Parr T. R., Jr, Bryan L. E. Persistence of Pseudomonas aeruginosa during ciprofloxacin therapy of a cystic fibrosis patient: transient resistance to quinolones and protein F-deficiency. J Antimicrob Chemother. 1990 Jun;25(6):995–1010. doi: 10.1093/jac/25.6.995. [DOI] [PubMed] [Google Scholar]
- Chapman J. S., Georgopapadakou N. H. Routes of quinolone permeation in Escherichia coli. Antimicrob Agents Chemother. 1988 Apr;32(4):438–442. doi: 10.1128/aac.32.4.438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chin N. X., Clynes N., Neu H. C. Resistance to ciprofloxacin appearing during therapy. Am J Med. 1989 Nov 30;87(5A):28S–31S. doi: 10.1016/0002-9343(89)90015-6. [DOI] [PubMed] [Google Scholar]
- Fukuda H., Hosaka M., Hirai K., Iyobe S. New norfloxacin resistance gene in Pseudomonas aeruginosa PAO. Antimicrob Agents Chemother. 1990 Sep;34(9):1757–1761. doi: 10.1128/aac.34.9.1757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Godfrey A. J., Hatlelid L., Bryan L. E. Correlation between lipopolysaccharide structure and permeability resistance in beta-lactam-resistant Pseudomonas aeruginosa. Antimicrob Agents Chemother. 1984 Aug;26(2):181–186. doi: 10.1128/aac.26.2.181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Godfrey A. J., Shahrabadi M. S., Bryan L. E. Distribution of porin and lipopolysaccharide antigens on a Pseudomonas aeruginosa permeability mutant. Antimicrob Agents Chemother. 1986 Nov;30(5):802–805. doi: 10.1128/aac.30.5.802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gotoh N., Nishino T. Decreases of the susceptibility to low molecular weight beta-lactam antibiotics in imipenem-resistant Pseudomonas aeruginosa mutants: role of outer membrane protein D2 in their diffusion. J Antimicrob Chemother. 1990 Feb;25(2):191–198. doi: 10.1093/jac/25.2.191. [DOI] [PubMed] [Google Scholar]
- Gotoh N., Wakebe H., Yoshihara E., Nakae T., Nishino T. Role of protein F in maintaining structural integrity of the Pseudomonas aeruginosa outer membrane. J Bacteriol. 1989 Feb;171(2):983–990. doi: 10.1128/jb.171.2.983-990.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hancock R. E., Benz R. Demonstration and chemical modification of a specific phosphate binding site in the phosphate-starvation-inducible outer membrane porin protein P of Pseudomonas aeruginosa. Biochim Biophys Acta. 1986 Sep 11;860(3):699–707. doi: 10.1016/0005-2736(86)90569-9. [DOI] [PubMed] [Google Scholar]
- Jap B. K. Molecular design of PhoE porin and its functional consequences. J Mol Biol. 1989 Jan 20;205(2):407–419. doi: 10.1016/0022-2836(89)90351-3. [DOI] [PubMed] [Google Scholar]
- Karkhanis Y. D., Zeltner J. Y., Jackson J. J., Carlo D. J. A new and improved microassay to determine 2-keto-3-deoxyoctonate in lipopolysaccharide of Gram-negative bacteria. Anal Biochem. 1978 Apr;85(2):595–601. doi: 10.1016/0003-2697(78)90260-9. [DOI] [PubMed] [Google Scholar]
- Marchou B., Bellido F., Charnas R., Lucain C., Pechère J. C. Contribution of beta-lactamase hydrolysis and outer membrane permeability to ceftriaxone resistance in Enterobacter cloacae. Antimicrob Agents Chemother. 1987 Oct;31(10):1589–1595. doi: 10.1128/aac.31.10.1589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muder R. R., Brennen C., Goetz A. M., Wagener M. M., Rihs J. D. Association with prior fluoroquinolone therapy of widespread ciprofloxacin resistance among gram-negative isolates in a Veterans Affairs medical center. Antimicrob Agents Chemother. 1991 Feb;35(2):256–258. doi: 10.1128/aac.35.2.256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mueller K., Ammann H. J., Doran D. M., Gerber P. R., Gubernator K., Schrepfer G. Structural databases and computer modeling in pharmaceutical research. Prog Clin Biol Res. 1989;291:219–226. [PubMed] [Google Scholar]
- Nikaido H., Nikaido K., Harayama S. Identification and characterization of porins in Pseudomonas aeruginosa. J Biol Chem. 1991 Jan 15;266(2):770–779. [PubMed] [Google Scholar]
- Nikaido H., Vaara M. Molecular basis of bacterial outer membrane permeability. Microbiol Rev. 1985 Mar;49(1):1–32. doi: 10.1128/mr.49.1.1-32.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oakley B. R., Kirsch D. R., Morris N. R. A simplified ultrasensitive silver stain for detecting proteins in polyacrylamide gels. Anal Biochem. 1980 Jul 1;105(2):361–363. doi: 10.1016/0003-2697(80)90470-4. [DOI] [PubMed] [Google Scholar]
- Quinn J. P., Dudek E. J., DiVincenzo C. A., Lucks D. A., Lerner S. A. Emergence of resistance to imipenem during therapy for Pseudomonas aeruginosa infections. J Infect Dis. 1986 Aug;154(2):289–294. doi: 10.1093/infdis/154.2.289. [DOI] [PubMed] [Google Scholar]
- Rubio T. T., Shapiro C. Ciprofloxacin in the treatment of Pseudomonas infection in cystic fibrosis patients. J Antimicrob Chemother. 1986 Nov;18 (Suppl 500):147–152. doi: 10.1093/jac/18.supplement_d.147. [DOI] [PubMed] [Google Scholar]
- Rådberg G., Nilsson L. E., Svensson S. Development of quinolone-imipenem cross resistance in Pseudomonas aeruginosa during exposure to ciprofloxacin. Antimicrob Agents Chemother. 1990 Nov;34(11):2142–2147. doi: 10.1128/aac.34.11.2142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Satake S., Yoshihara E., Nakae T. Diffusion of beta-lactam antibiotics through liposome membranes reconstituted from purified porins of the outer membrane of Pseudomonas aeruginosa. Antimicrob Agents Chemother. 1990 May;34(5):685–690. doi: 10.1128/aac.34.5.685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spratt B. G. Properties of the penicillin-binding proteins of Escherichia coli K12,. Eur J Biochem. 1977 Jan;72(2):341–352. doi: 10.1111/j.1432-1033.1977.tb11258.x. [DOI] [PubMed] [Google Scholar]
- Towbin H., Staehelin T., Gordon J. Electrophoretic transfer of proteins from polyacrylamide gels to nitrocellulose sheets: procedure and some applications. Proc Natl Acad Sci U S A. 1979 Sep;76(9):4350–4354. doi: 10.1073/pnas.76.9.4350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trias J., Nikaido H. Outer membrane protein D2 catalyzes facilitated diffusion of carbapenems and penems through the outer membrane of Pseudomonas aeruginosa. Antimicrob Agents Chemother. 1990 Jan;34(1):52–57. doi: 10.1128/aac.34.1.52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trias J., Nikaido H. Protein D2 channel of the Pseudomonas aeruginosa outer membrane has a binding site for basic amino acids and peptides. J Biol Chem. 1990 Sep 15;265(26):15680–15684. [PubMed] [Google Scholar]
- Trias J., Rosenberg E. Y., Nikaido H. Specificity of the glucose channel formed by protein D1 of Pseudomonas aeruginosa. Biochim Biophys Acta. 1988 Mar 3;938(3):493–496. doi: 10.1016/0005-2736(88)90148-4. [DOI] [PubMed] [Google Scholar]
- Wolfson J. S., Hooper D. C. Fluoroquinolone antimicrobial agents. Clin Microbiol Rev. 1989 Oct;2(4):378–424. doi: 10.1128/cmr.2.4.378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woodruff W. A., Hancock R. E. Pseudomonas aeruginosa outer membrane protein F: structural role and relationship to the Escherichia coli OmpA protein. J Bacteriol. 1989 Jun;171(6):3304–3309. doi: 10.1128/jb.171.6.3304-3309.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamano Y., Nishikawa T., Komatsu Y. Outer membrane proteins responsible for the penetration of beta-lactams and quinolones in Pseudomonas aeruginosa. J Antimicrob Chemother. 1990 Aug;26(2):175–184. doi: 10.1093/jac/26.2.175. [DOI] [PubMed] [Google Scholar]
- Yoshihara E., Nakae T. Identification of porins in the outer membrane of Pseudomonas aeruginosa that form small diffusion pores. J Biol Chem. 1989 Apr 15;264(11):6297–6301. [PubMed] [Google Scholar]
- Yoshimura F., Zalman L. S., Nikaido H. Purification and properties of Pseudomonas aeruginosa porin. J Biol Chem. 1983 Feb 25;258(4):2308–2314. [PubMed] [Google Scholar]