Abstract
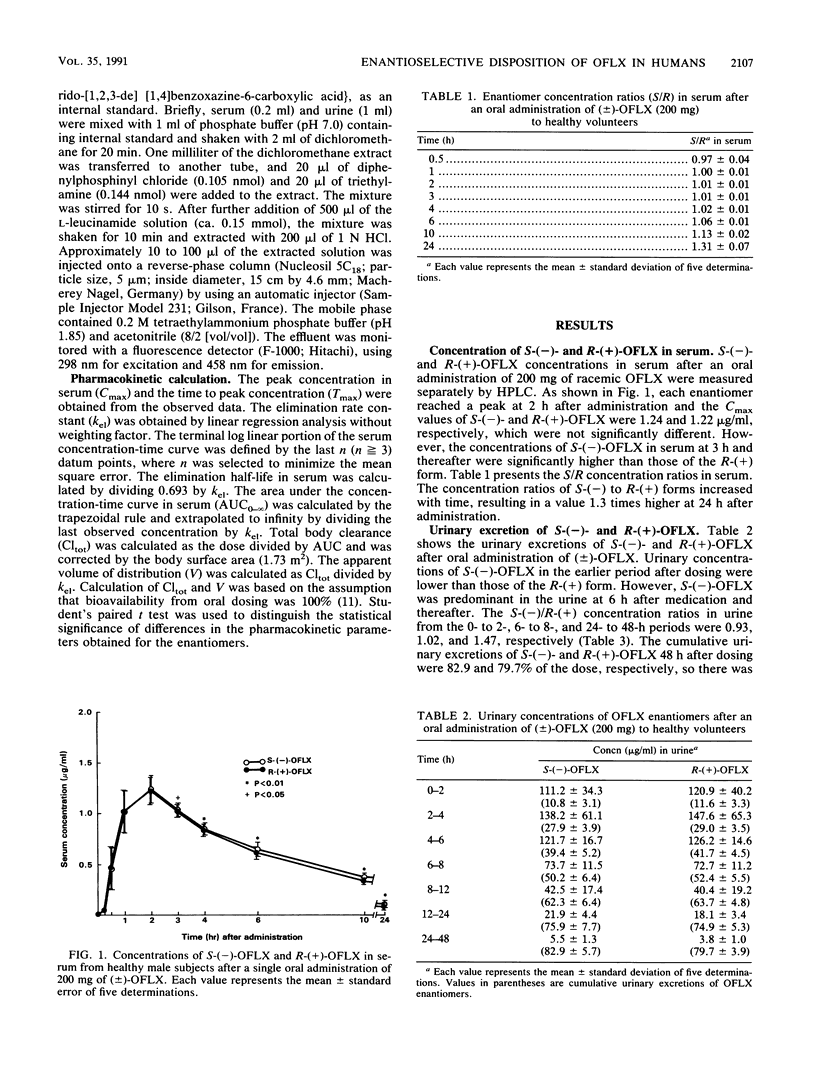
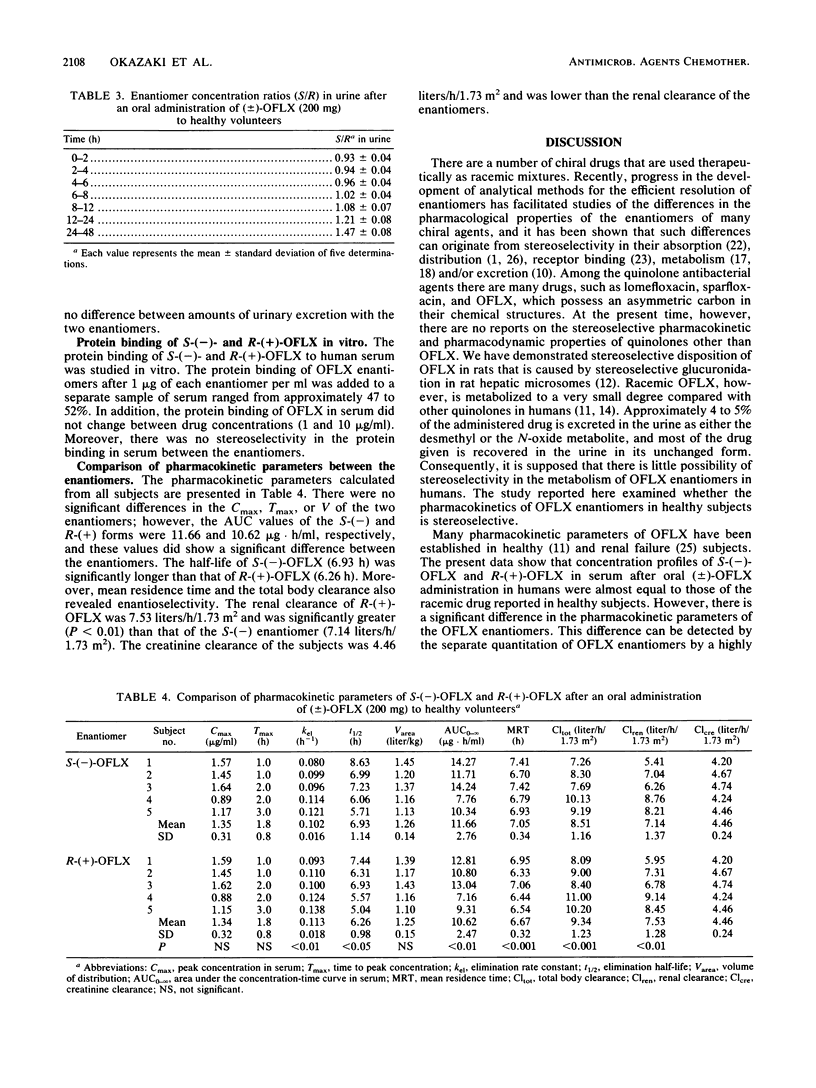
The enantioselective disposition of ofloxacin (OFLX) was studied in healthy subjects after oral administration of (+/-)-OFLX at a dose of 200 mg. S-(-)-OFLX and R-(+)-OFLX concentrations in serum and urine were measured separately by high-performance liquid chromatography, and various pharmacokinetic parameters were calculated from the data. The ratio of S-(-) to R-(+) enantiomer concentrations in serum showed a increase with time, with S/R ratios of 1.01 at 2 h and 1.31 at 24 h. The terminal elimination half-life of S-(-)-OFLX was 6.9 h, which was significantly greater (P less than 0.05) than that of the R-(+) enantiomer (6.3 h). S-(-)-OFLX also revealed a significantly greater area under the concentration-time curve in serum, mean residence time, and total body clearance than the R-(+) enantiomer did. The renal clearance of S-(-)-OFLX (7.14 liters/h/1.73 m2) was significantly lower than that of the R-(+) enantiomer (7.53 liters/h/1.73 m2). Although the difference in the pharmacokinetic parameters of the enantiomers was small, their disposition in humans was found to be stereoselective. The difference between the enantiomers may be explained by the difference in their renal excretion.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Albani F., Riva R., Contin M., Baruzzi A. Stereoselective binding of propranolol enantiomers to human alpha 1-acid glycoprotein and human plasma. Br J Clin Pharmacol. 1984 Aug;18(2):244–246. doi: 10.1111/j.1365-2125.1984.tb02462.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eichelbaum M., Mikus G., Vogelgesang B. Pharmacokinetics of (+)-, (-)- and (+/-)-verapamil after intravenous administration. Br J Clin Pharmacol. 1984 Apr;17(4):453–458. doi: 10.1111/j.1365-2125.1984.tb02371.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fujimoto T., Mitsuhashi S. In vitro antibacterial activity of DR-3355, the S-(-)-isomer of ofloxacin. Chemotherapy. 1990;36(4):268–276. doi: 10.1159/000238777. [DOI] [PubMed] [Google Scholar]
- Hayakawa I., Atarashi S., Yokohama S., Imamura M., Sakano K., Furukawa M. Synthesis and antibacterial activities of optically active ofloxacin. Antimicrob Agents Chemother. 1986 Jan;29(1):163–164. doi: 10.1128/aac.29.1.163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoshino K., Sato K., Une T., Osada Y. Inhibitory effects of quinolones on DNA gyrase of Escherichia coli and topoisomerase II of fetal calf thymus. Antimicrob Agents Chemother. 1989 Oct;33(10):1816–1818. doi: 10.1128/aac.33.10.1816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Igwemezie L., Kerr C. R., McErlane K. M. The pharmacokinetics of the enantiomers of mexiletine in humans. Xenobiotica. 1989 Jun;19(6):677–682. doi: 10.3109/00498258909042305. [DOI] [PubMed] [Google Scholar]
- Kurrle E., Dekker A. W., Gaus W., Haralambie E., Krieger D., Rozenberg-Arska M., de Vries-Hospers H. G., van der Waaij D., Wendt F. Prevention of infection in acute leukemia: a prospective randomized study on the efficacy of two different drug regimens for antimicrobial prophylaxis. Infection. 1986 Sep-Oct;14(5):226–232. doi: 10.1007/BF01644268. [DOI] [PubMed] [Google Scholar]
- Küpfer A., Desmond P. V., Schenker S., Branch R. A. Stereoselective metabolism and disposition of the enantiomers of mephenytoin during chronic oral administration of the racemic drug in man. J Pharmacol Exp Ther. 1982 Jun;221(3):590–597. [PubMed] [Google Scholar]
- Lehr K. H., Damm P. Quantification of the enantiomers of ofloxacin in biological fluids by high-performance liquid chromatography. J Chromatogr. 1988 Mar 4;425(1):153–161. doi: 10.1016/0378-4347(88)80015-x. [DOI] [PubMed] [Google Scholar]
- Lennard M. S., Tucker G. T., Silas J. H., Freestone S., Ramsay L. E., Woods H. F. Differential stereoselective metabolism of metoprolol in extensive and poor debrisoquin metabolizers. Clin Pharmacol Ther. 1983 Dec;34(6):732–737. doi: 10.1038/clpt.1983.242. [DOI] [PubMed] [Google Scholar]
- Lode H., Höffken G., Olschewski P., Sievers B., Kirch A., Borner K., Koeppe P. Pharmacokinetics of ofloxacin after parenteral and oral administration. Antimicrob Agents Chemother. 1987 Sep;31(9):1338–1342. doi: 10.1128/aac.31.9.1338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okazaki O., Kurata T., Hakusui H., Tachizawa H. Stereoselective glucuronidation of ofloxacin in rat liver microsomes. Drug Metab Dispos. 1991 Mar-Apr;19(2):376–380. [PubMed] [Google Scholar]
- Okazaki O., Kurata T., Tachizawa H. Stereoselective metabolic disposition of enantiomers of ofloxacin in rats. Xenobiotica. 1989 Apr;19(4):419–429. doi: 10.3109/00498258909042283. [DOI] [PubMed] [Google Scholar]
- Silber B., Holford N. H., Riegelman S. Stereoselective disposition and glucuronidation of propranolol in humans. J Pharm Sci. 1982 Jun;71(6):699–704. doi: 10.1002/jps.2600710623. [DOI] [PubMed] [Google Scholar]
- Sisenwine S. F., Tio C. O., Hadley F. V., Liu A. L., Kimmel H. B., Ruelius H. W. Species-related differences in the stereoselective glucuronidation of oxazepam. Drug Metab Dispos. 1982 Nov-Dec;10(6):605–608. [PubMed] [Google Scholar]
- Sudo K., Okazaki O., Tsumura M., Tachizawa H. Isolation and identification of metabolites of ofloxacin in rats, dogs and monkeys. Xenobiotica. 1986 Aug;16(8):725–732. doi: 10.3109/00498258609043563. [DOI] [PubMed] [Google Scholar]
- Tamai I., Ling H. Y., Timbul S. M., Nishikido J., Tsuji A. Stereospecific absorption and degradation of cephalexin. J Pharm Pharmacol. 1988 May;40(5):320–324. doi: 10.1111/j.2042-7158.1988.tb05259.x. [DOI] [PubMed] [Google Scholar]
- Wade D. N., Mearrick P. T., Morris J. L. Active transport of L-dopa in the intestine. Nature. 1973 Apr 13;242(5398):463–465. doi: 10.1038/242463a0. [DOI] [PubMed] [Google Scholar]
- Weiland G. A., Oswald R. E. The mechanism of binding of dihydropyridine calcium channel blockers to rat brain membranes. J Biol Chem. 1985 Jul 15;260(14):8456–8464. [PubMed] [Google Scholar]
- White L. O., MacGowan A. P., Lovering A. M., Reeves D. S., Mackay I. G. A preliminary report on the pharmacokinetics of ofloxacin, desmethyl ofloxacin and ofloxacin N-oxide in patients with chronic renal failure. Drugs. 1987;34 (Suppl 1):56–61. doi: 10.2165/00003495-198700341-00013. [DOI] [PubMed] [Google Scholar]
- White L. O., MacGowan A. P., Mackay I. G., Reeves D. S. The pharmacokinetics of ofloxacin, desmethyl ofloxacin and ofloxacin N-oxide in haemodialysis patients with end-stage renal failure. J Antimicrob Chemother. 1988 Sep;22 (Suppl 100):65–72. doi: 10.1093/jac/22.supplement_c.65. [DOI] [PubMed] [Google Scholar]
- Yacobi A., Levy G. Protein binding of warfarin enantiomers in serum of humans and rats. J Pharmacokinet Biopharm. 1977 Apr;5(2):123–131. doi: 10.1007/BF01066216. [DOI] [PubMed] [Google Scholar]
- Yoshikawa T., Muranushi N., Yoshida M., Oguma T., Hirano K., Yamada H. Transport characteristics of ceftibuten (7432-S), a new oral cephem, in rat intestinal brush-border membrane vesicles: proton-coupled and stereoselective transport of ceftibuten. Pharm Res. 1989 Apr;6(4):302–307. doi: 10.1023/a:1015994323639. [DOI] [PubMed] [Google Scholar]


