Abstract
OBJECTIVE—We have developed a novel platform for display and delivery of bioactive peptides that links the biological properties of the peptide to the pharmacokinetic properties of an antibody. Peptides engineered in the MIMETIBODY platform have improved biochemical and biophysical properties that are quite distinct from those of Fc-fusion proteins. CNTO736 is a glucagon-like peptide 1 (GLP-1) receptor agonist engineered in our MIMETIBODY platform. It retains many activities of native GLP-1 yet has a significantly enhanced pharmacokinetic profile. Our goal was to develop a long-acting GLP-1 receptor agonist with sustained efficacy.
RESEARCH DESIGN AND METHODS—In vitro and in vivo activity of CNTO736 was evaluated using a variety of rodent cell lines and diabetic animal models.
RESULTS—Acute pharmacodynamic studies in diabetic rodents demonstrate that CNTO736 reduces fasting and postprandial glucose, decreases gastric emptying, and inhibits food intake in a GLP-1 receptor–specific manner. Reduction of food intake following CNTO736 dosing is coincident with detection of the molecule in the circumventricular organs of the brain and activation of c-fos in regions protected by the blood-brain barrier. Diabetic rodents dosed chronically with CNTO736 have lower fasting and postprandial glucose and reduced body weight.
CONCLUSIONS—Taken together, our data demonstrate that CNTO736 produces a spectrum of GLP-1 receptor–dependent actions while exhibiting significantly improved pharmacokinetics relative to the native GLP-1 peptide.
Drug development strategies for therapeutic peptides continue to be challenging despite advances in technologies such as pegylation and lipidation (1–4). Although important biological processes are regulated by peptides, successful development of peptide drugs has been limited and transformation of a metabolically labile peptide into a drug remains challenging. In contrast, considerable advances have been made in the development of antibody therapeutics (5,6). A technology that could link the activity of a target peptide with the pharmacokinetic characteristics of an antibody would be a valuable addition to tools available for drug discovery. To address this need, we developed the MIMETIBODY platform as a novel technology for the display and delivery of bioactive peptides. Using protein design tools, we linked an antibody Fc domain to a bioactive glucagon-like peptide 1 (GLP-1) analog and engineered the construct for optimal biochemical and biophysical properties.
GLP-1 is a 30–amino acid peptide secreted from L-cells of the intestine following nutrient ingestion (7–10). GLP-1 is rapidly degraded in vivo with a half-life of <2 min and cleared via the kidney (11,12). When circulating glucose concentrations are elevated, GLP-1 increases insulin and decreases glucagon secretion from the pancreas and slows gastric emptying, thereby reducing glucose appearance in the circulation and enhancing glucose clearance from the circulation (13–15). In rodent models, GLP-1 expands β-cell mass via induction of β-cell proliferation and neogenesis and reduction of β-cell apoptosis (16–20). The cytoprotective actions of GLP-1 also promote survival of human islets (21,22). Furthermore, GLP-1 reduces food intake, and therapy with GLP-1 receptor agonists has been associated with weight loss in clinical studies (23,24). Thus, GLP-1 receptor agonists are attractive therapeutic candidates for the treatment of type 2 diabetes.
CNTO736 is a GLP-1 receptor agonist engineered in our MIMETIBODY platform that incorporates a GLP-1 peptide analogue genetically fused to a domain that includes the Fc portion of an antibody (25,26). In addition to an amino acid substitution in the peptide rendering it resistant to dipeptidyl peptidase IV (27,28), the increased molecular weight and pharmacokinetic properties of an Fc were expected to enable sustained delivery of a GLP-1 receptor agonist. We demonstrate that CNTO736 dose-dependently increases cAMP and insulin secretion from islets in a glucose-dependent manner. In rodent models of type 2 diabetes, acute dosing with CNTO736 lowers fasting and postprandial blood glucose with a significantly longer duration of action than native GLP-1, and chronic dosing with CNTO736 decreases body weight. Although CNTO736 is a large molecule that is not likely to efficiently cross the blood-brain barrier, it can be detected in the circumventricular organs of the brain following peripheral dosing, and c-fos expression is detected in regions that are protected by the blood-brain barrier. Food intake is reduced in mice and rats following peripheral dosing with CNTO736, correlating with the appearance of the molecule in the hypothalamus. Hence, the generation of stable bioactive peptide therapeutics with optimized pharmacokinetic properties may provide a new option for the treatment of metabolic disorders.
RESEARCH DESIGN AND METHODS
Animal studies.
All animal studies were performed according to the National Research Council's Guide for the Care and Use of Laboratory Animals and were approved by the internal institutional animal care and use committee. db/db mice were purchased from The Jackson Laboratories. For diet-induced obesity (DIO) mice experiments, C57BL/6J mice were maintained on a diet containing 60.9% kcal from fat from 4 weeks of age, and all animals achieved 3 consecutive weeks of diabetic fasting blood glucose (FBG) values (>120 mg/dl).
Expression and purification of CNTO736.
CNTO736 was constructed by fusing a GLP-1 peptide analogue to a flexible Gly/Ser linker and a fragment of a V region heavy chain (VH) domain linked directly to the CH2 and CH3 domains of an Fc. The amino acid sequence is shown in Fig. 1A. Genes encoding CNTO736 or CNTO1996, a negative control molecule that lacks a GLP-1 segment, were cloned into a vector for mammalian expression under control of the cytomegalovirus promoter. For transient expression, HEK 293E cells were expanded (Dulbecco's modified Eagle's medium [Invitrogen] + 10% fetal bovine serum) and used to seed a 10-tier cell factory (5 × 107 cells in growth medium [1,200 ml]). Twenty-four hours later, the cells were transfected. The transfection mix was replaced with 293-SFMII medium (Invitrogen) with 5 mmol/l sodium butyrate (1,200 ml) after 24 h. Four days later, the conditioned medium was harvested, filtered, and stored at 4°C. CNTO736 was purified using a Protein A MabSelect column (GE Healthcare) and Immunopure Gentle Ag/Ab binding and elution buffers (Pierce). The purified product was dialyzed into 20 mmol/l Tris, pH 7.4. The final column was a Superdex 200 column (GE Healthcare) in PBS. Selected fractions were pooled and concentrated.
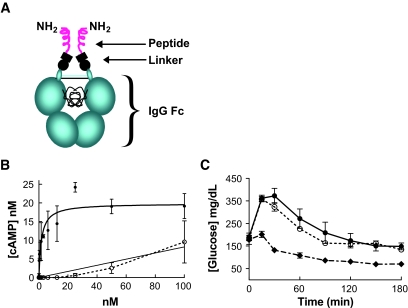
FIG. 1.
CNTO736 has improved activity relative to constructs lacking a linker. A: The schematic outlines the structure of CNTO736 including the CH2 and CH3 domains of the Fc, the hinge including the disulfide bonds, a linker containing a partial VH region, and two GLP-1 peptides. The amino acid sequence of CNTO736 beginning at the NH2-terminus is as follows: HSEGTFTSDVSSYLEGQAAKEFIAWLVKGRGGGSGGGSGTLVTVSSESKYGPPCPPCPAPEAA… Fc B. The concentration of cAMP was measured in INS-1E cells after addition of increasing concentrations of CNTO736 or a molecule lacking the hinge. The fit to the CNTO736 data provided an EC50 of 1.8 nmol/l. C: Fasted mice (DIO, n = 5) were dosed intravenously with vehicle (▪), CNTO736 (0.5 mg/kg) (♦), or a construct lacking a hinge (0.5 mg/kg) (○) 10 min before an ipGTT. The results are presented as the means ± SE (n = 5).
Cells and islets.
INS-1E cells were kindly provided by C.B. Wollheim. Human and Sprague-Dawley rat islets were isolated using an enzymatic digestion and density gradient purification as described (29).
cAMP.
INS-1E cells were plated in 96-well plates (1 × 105 cells/well) in RPMI-1640 (Invitrogen) containing fetal bovine serum (10%), l-glutamine (1%), sodium pyruvate (1%), nonessential amino acids (1%), and β-mercaptoethanol (50 μmol/l). The cells recovered for 4 days at 37°C with 5% CO2. Media was aspirated from the wells, and 24 μl of Alexa Fluor 647 anti-cAMP antibody (LANCE cAMP Kit; Perkin Elmer) was added followed by 24 μl of CNTO736 or a construct lacking a linker (in PBS/0.5% BSA/0.5 mmol/l isobutylmethylxanthine). The cells were stimulated at room temperature for 7 min and lysed per the manufacture's protocol (Perkin Elmer). The plates were incubated at room temperature for 1 h, and the fluorescence intensity was measured at 665 nm. cAMP concentrations were determined using a standard curve.
Insulin secretion from rat or human islets.
Islets were suspended at a density of 20 islets/ml in functionality/viability media (MediaTech) containing BSA (1%), l-glutamine (1%), penicillin/streptomycin (1%), and glucose (0.5 mmol/l). Approximately 20 islets were added to each well of a 24-well plate and incubated at 37°C in 5% CO2. After 2 h, functionality/viability media containing BSA (1%), l-glutamine (1%), penicillin/streptomycin (1%), CNTO736 (50 nmol/l), and glucose (3.5 or 15 mmol/l) was added. Supernatant was collected at baseline and after 4 h and was stored at −20°C. Rat insulin was quantitated using an ultrasensitive enzyme-linked immunosorbent assay (ELISA) (Crystal Chem), and human insulin was quantitated by ELISA (Linco Research).
Pharmacokinetics of CNTO736 in mice.
C57BL/6 mice were dosed intravenously with CNTO736 (1 mg/kg) or GLP-1 peptide (0.062 mg/kg; Sigma). At various times, three animals were killed and blood was collected via cardiac puncture in 3.8% sodium citrate containing protease inhibitors (Roche Complete EDTA free; Roche Applied Science). Plasma was isolated and stored at −80°C. The concentration of intact CNTO736 was measured using a modified form of a purchased ELISA designed to detect intact GLP-1 (Linco). Briefly, CNTO736 was captured as described in the protocol, but a goat anti-human H + L alk-phos conjugate (Jackson Immunoresearch) was used to detect intact CNTO736. Fluorescence was read and the data analyzed with Softmax-Pro (Molecular Devices).
Intraperitoneal insulin glucose test in DIO mice.
Male DIO mice were randomized (n = 5) based on FBG. Mice were dosed intravenously with CNTO736, the negative control lacking the GLP-1 peptide, or PBS 10 min before an intraperitoneal glucose tolerance test (ipGTT).
ipGTT and food intake in GLP-1 receptor knockout mice.
Fasted male GLP-1 receptor knockout mice (n = 5) (30) and C57BL/6 age-matched controls (n = 6) were dosed intravenously with CNTO736 (1 mg/kg) or a negative control lacking the GLP-1 peptide. An ipGTT was done 30 min after dosing. In a separate study, fasted male GLP-1 receptor knockout mice (n = 5) and C57BL/6 age-matched controls (n = 6) were dosed subcutaneously with CNTO736 (1 mg/kg) or a negative control lacking the GLP-1 peptide, and food intake was measured over the next 24 h.
Gastric emptying in wild-type mice.
Mice (n = 5) were fasted overnight (16–18 h), and fed preweighed food in the morning. After 1 h of refeeding, the remaining food was weighed for measurement of food intake, and mice were dosed intravenously with CNTO736 (2.4 mg/kg) or PBS (31). The animals were deprived of food for the remainder of the study. Four hours postdosing, the stomach was exposed by laparotomy, quickly ligated at both the pylorus and cardia, and removed. The wet weight of the stomach content was determined, and the amount of food retained in the stomach was calculated.
Immunohistochemistry of anti-human IgG in brain sections of rats.
Sprague-Dawley rats (n = 3) were fasted for 24 h and dosed intravenously with PBS or CNTO736 (1 mg/kg) just before the dark cycle. After 30 min, 2 h, and 6 h, food and water intake measurements were recorded and rats were anesthetized with pentobarbital. Transcardial perfusion included PBS followed by paraformaldehyde (PFA) (4%) with sucrose. Following decapitation, rat skulls were stored overnight in PFA (4%) at 4°C. Brains were dissected, stored in PBS, and shipped to NeuroScience Associates (Knoxville, TN) for immunohistochemistry analysis. A multibrain block was prepared using MultiBrain Technology (NeuroScience Associates), frozen by immersion in chilled isopentane, and mounted on a freezing stage of an AO 860 sliding microtome. The MultiBrain block was sectioned coronally at 35 μ with sequential collection into a 4 × 5 array of containers filled with antigen preserve solution (50% PBS, pH 7.0; 50% ethylene glycol, 1% polyvinyl pyrrolidone). Sections were stained with anti-human Fc (Jackson ImmunoResearch) and anti–c-fos (Calbiochem) reagents for CNTO736 and c-fos, respectively. Antibody binding was visualized using appropriate secondary antibodies, avidin–horseradish peroxidase and diaminobenzidine tetrahydrochloride with hydrogen peroxide.
Chronic dosing in DIO mice.
Male DIO mice (n = 7) were subcutaneously dosed daily with vehicle or CNTO736 (0.1 and 1 mg/kg) for 6 weeks. FBG was measured from tail vein blood using a hand-held glucometer (Lifescan) twice per week. Body weights were measured daily during the 6 weeks of dosing. Dual-energy X-ray absorptiometry analysis was done at the end of the 6-week study on the CNTO736 (1 mg/kg) and vehicle-treated groups.
Statistical analysis.
All values are presented as the means ± SE. Comparisons among groups were made using ANOVA followed by unpaired, nonparametric two-tailed Student's t test. Differences were considered statistically significant at P < 0.05.
RESULTS
MIMETIBODY engineering.
Various constructs were engineered to balance optimization of GLP-1 activity with maximal in vivo stability, concentrating the design on the linker because it is such a critical element. The linker must be flexible enough to enable effective engagement of the GLP-1 receptor yet stable enough to maintain in vivo attachment to the Fc portion of the molecule since it is responsible for imparting extended pharmacokinetic properties (Fig. 1A). To illustrate the importance of the linker, constructs with different linkers were evaluated. One variant in which the GLP-1 segment was linked directly to the Fc hinge had improved pharmacokinetic properties but was linked to significant decreases in receptor activity (Fig. 1B) and in vivo efficacy (Fig. 1C). The optimal variant, CNTO736, maintained the bioactivity of GLP-1, while extending the half life ∼1,000-fold compared with GLP-1 in mice (30 h vs. 1–2 min) (12).
In vitro characterization.
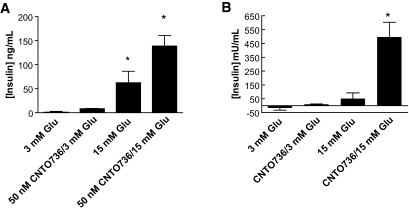
CNTO736 increased cAMP accumulation in INS-1 cells in a dose-dependent manner with an EC50 of 4.9 ± 2.5 nmol/l (n = 6) compared with 0.2 ± 0.1 nmol/l (n = 5) for native GLP-1 (Fig. 1B shows one representative dataset). To directly compare the affinity of CNTO736 and GLP-1 binding to the GLP-1 receptor, competitive binding experiments using 125I-labeled GLP-1 demonstrated that binding of CNTO736 to GLP-1 receptor on INS-1 cells was approximately eightfold weaker than native GLP-1 (data not shown). Both the cAMP and binding studies indicate that addition of the hinge and Fc onto the GLP-1 peptide results in a decreased affinity for the receptor. Nevertheless, CNTO736 stimulated insulin secretion from INS-1 cells with an EC50 of 0.03 nmol/l (data not shown) and enhanced insulin secretion from rat and human islets in a glucose-dependent manner (Fig. 2A and B).
FIG. 2.
CNTO736 induces glucose-dependent insulin secretion. Rat islets (A) or human islets (B) were incubated with CNTO736 (50 nmol/l) in the presence of low or high glucose, and the concentration of secreted insulin was measured after 4 h.
ipGTT in DIO mice.
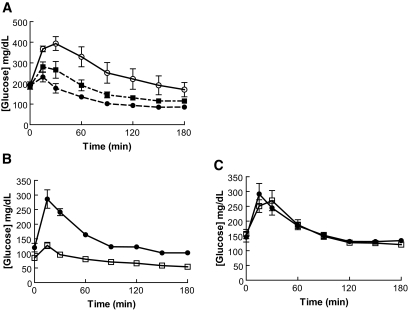
The in vivo activity of CNTO736 was evaluated in an acute study to ascertain whether the molecule was able to reduce blood glucose levels. CNTO736 or a negative control lacking the GLP-1 peptide was dosed intravenously to DIO mice, and an ipGTT was performed 10 min later. CNTO736 reduced the glucose excursion in a dose-dependent manner (Fig. 3A). At 20 μg/kg CNTO736, the glucose area under the curve was reduced >35% relative to control animals, while the glucose area under the curve for mice treated with 100 μg/kg was reduced by 53%.
FIG. 3.
CNTO736 improves glucose tolerance in normal and DIO mice but not GLP-1 receptor knockout mice. A: Fasted mice (DIO, n = 5) were dosed intravenously with CNTO736 [0.02 (▪) and 0.1 (•) mg/kg] or the negative control lacking the GLP-1 peptide (○) 10 min before an ipGTT. B: Fasted mice (C57BL/6, n = 6) were dosed intravenously with CNTO736 (1 mg/kg) (□) or the negative control (1 mg/kg) (•) 30 min before an ipGTT. C: Fasted mice (GLP-1 receptor knockout, n = 5) were dosed intravenously with CNTO736 (1 mg/kg) (□) or control (1 mg/kg) (•) 30 min before an ipGTT. The results are presented as the means ± SE (n = 5).
ipGTT in wild-type and GLP-1 receptor knockout mice.
To determine whether the glucoregulatory actions of CNTO736 were GLP-1 receptor dependent, we evaluated the activity of CNTO736 in GLP-1 receptor knockout (Glp1r−/−) mice (30). As previously demonstrated in DIO mice, wild-type mice dosed with CNTO736 (1 mg/kg) had significantly reduced circulating glucose following an ipGTT (Fig. 3B), while no change in blood glucose was observed following CNTO736 administration in Glp1r−/− mice (Fig. 3C). In addition, FBG was reduced significantly 30 min after administration of CNTO736 in wild-type mice (85 ± 13 vs. 120 ± 25 mg/dl) but not in Glp1r−/− mice (156 ± 38 vs. 145 ± 37 mg/dl).
Food intake in wild-type and GLP-1 receptor knockout mice and gastric emptying in wild-type mice.
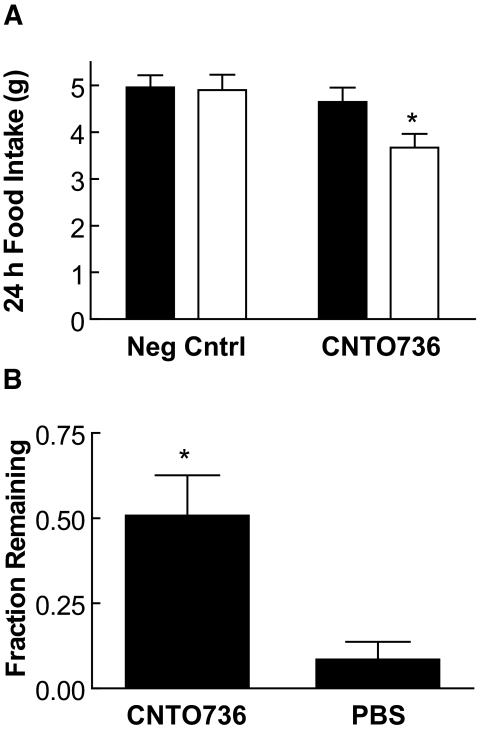
Treatment with CNTO736 (1 mg/kg) reduced 24-h food intake by 25% in wild-type, but not in Glp1r−/−, mice (Fig. 4A). In a separate study, gastric emptying was estimated by weighing stomach contents after mice were given a preweighed meal. There was a fivefold increase in retained stomach contents in animals dosed with CNTO736 relative to control animals (Fig. 4B).
FIG. 4.
CNTO736 decreases food intake and delays gastric emptying. A: Fasted GLP-1 receptor knockout (n = 5) (▪) or C57BL/6 mice (n = 6) (□) were dosed subcutaneously with CNTO736 (1 mg/kg) or a negative control (1 mg/kg). Food intake was monitored over 24 h. The results are presented as the means ± SE (n = 5). *P value <0.05. B: Fasted mice (n = 5) were given a preweighed meal and the amount of food consumed in 1 h was measured. The mice were dosed intravenously with CNTO736 (2.4 mg/kg) or vehicle and were killed after 4 h. The wet weight of the stomach contents was measured to determine what percent of the food remained in the stomach as an estimate of gastric emptying. The results are presented as means ± SE (n = 10). *P value <0.05.
Fc and c-fos immunohistochemistry in brain sections of wild-type rats.
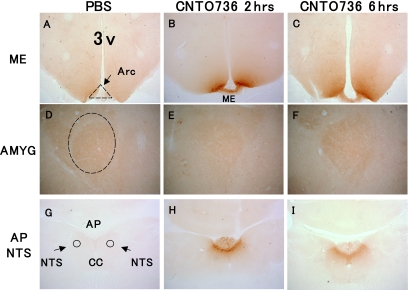
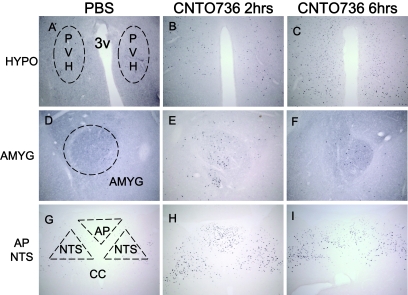
Some actions of small peptidic GLP-1 receptor agonists have been attributed to activation of central receptors that regulate food intake and satiety. We anticipated that the molecular weight of CNTO736 would inhibit its passage across the blood-brain barrier and limit its ability to regulate food intake. To assess whether peripherially administered CNTO736 could access central GLP-1 receptors and directly activate c-fos expression in the brain, rats were dosed intravenously with CNTO736 and brains were isolated 2 or 6 h after dosing. Brains were sectioned and stained for the presence of human Fc. CNTO736 was detected in the median eminence and area postrema of the nucleus of the solitary tract at both the 2- and 6-h time points but not in the central nucleus of the amygdale (Fig. 5A–I). However, c-fos expression could be detected via immunohistochemistry in the central nucleus of the amygdale as well as in the hypothalamus and area postrema (Fig. 6A–I). The appearance of CNTO736 in the median eminence and area postrema and c-fos expression in the hypothalamus, area postrema, and amygdale correlated with reductions in food and water intake (data not shown).
FIG. 5.
CNTO736 is present in the area of the hypothalamus and circumventricular organs but not in the amygdale following peripheral dosing. Brain sections from rats (n = 3) dosed intravenously with PBS (A, D, and G), CNTO736 for 2 h (B, E, and H) and CNTO736 for 6 h (C, F, and I). Brain sections were taken at the level of the median eminence (ME) (A–C), central nucleus of the amygdale (AMYG) (D–F), and area postrema (AP), including nucleus of the solitary tract (NTS) (G–I). Coronal brain sections were stained with an anti-human IgG antibody to detect the presence of CNTO736. The images are representative of sections taken from three rats per group. Picture magnification was 4× for A–C and G–I and 10× for D–F. Staining in PBS treated animals at 2 and 6 h postinjection were identical. Additional abbreviations include central canal (CC), third ventricle (3v), and arcuate nucleus (Arc).
FIG. 6.
Peripheral treatment of CNTO736 leads to neuronal activation of several brain areas as measured by c-fos immunohistochemical staining. Brain sections from rats (n = 3) dosed intravenously with PBS (A, D, and G), CNTO736 for 2 h (B, E, and H), and CNTO736 for 6 h (C, F, and I). Brain sections were taken at the level of the hypothalamus (HYPO) (A–C), central nucleus of the amygdale (AMYG) (D–F), area postrema (AP), and nucleus of the solitary tract (NTS) (G–I). The images are representative of sections taken from three rats per group. Coronal brain sections were stained with an anti–c-fos antibody. All pictures were taken at a ×10 magnification. Staining of PBS-treated animals at 2 and 6 h postinjection were identical. PVH, paraventricular nucleus of the hypothalamus.
Chronic dosing in DIO mice.
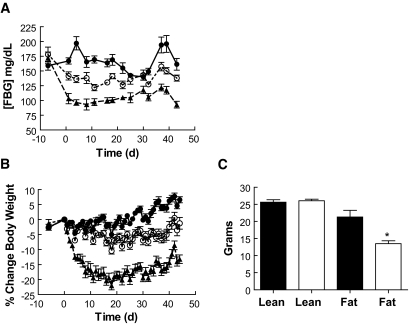
DIO mice dosed chronically with CNTO736 demonstrated a dose-dependent reduction in FBG (Fig. 7A). In addition, treatment with CNTO736 over a 6-week period reduced body weight by 7.8 or 19.8% relative to control mice after 6 weeks for the 0.1 or 1 mg/kg doses, respectively (Fig. 7B). Dual-energy X-ray absorptiometry analysis indicated that the reduction in body weight was primarily due to a loss of fat mass, while muscle mass was unchanged (Fig. 7C).
FIG. 7.
Chronic CNTO736 dosing improves glucose control and decreases body weight in DIO mice. CNTO736 (0.1 mg/kg) (□) and (1 mg/kg) (▴) or vehicle (•) was dosed subcutaneously daily for 6 weeks (n = 7). A: Fasting blood glucose. B: Body weight. C: Dual-energy X-ray analysis for lean and fat mass in the vehicle (▪) and CNTO736 groups (1 mg/kg) (□). The results are presented as the means ± SE (n = 5). *P value <0.05.
DISCUSSION
Protein therapeutics represent the fastest-growing segment in the pharmaceutical industry. The increasing success rate of antibody therapeutics has fueled an interest in expanding the utility of protein therapeutics to address other unmet medical needs (32). Peptides represent a rich source of therapeutic targets, but unmodified peptides have not traditionally made good drugs because of their high rates of metabolism and clearance. A technology that could couple the desirable pharmacological properties of antibodies with the bioactivity of small peptides would be a valuable extension of antibody technology. The MIMETIBODY platform was developed to address the gap between large protein and small peptide molecules. A variety of peptides were engineered into the platform to demonstrate the utility and versatility of the platform. This novel class of proteins provides a scaffold for display and delivery of bioactive peptides that is unique from other fusion protein technologies. The placement of the genetic fusion and the nature of the linker sequence are critical for maintenance of peptide activity. During engineering of CNTO736, we have tested a construct in which the GLP-1 moiety was fused directly to the hinge of the Fc; this construct exhibited very low activity (Fig. 1B). Similarly, others (31,33) have reported that fusion of a GLP-1 analogue to an IgG1 Fc or albumin results in nearly 100-fold lower activity. Thus, optimization of the linker sequence is an essential component of the MIMETIBODY technology.
We have used our novel MIMETIBODY platform to engineer a molecule with potential for application to type 2 diabetes. GLP-1 receptor agonists have gained significant attention because of several attractive properties of the peptide. GLP-1 stimulates insulin release from β-cells in a glucose-dependent manner (7,10,34), increases insulin biosynthesis in the β-cell (7), regulates gastric emptying (13–15), inhibits food intake (35–37), and increases β-cell mass in rodents (16,17,19–21). However, GLP-1 itself is not a desirable therapeutic because of its extremely short in vivo half-life (T1/2 = ∼1–2 min) (11,12). Our data demonstrate that CNTO736 maintains the bioactivity properties of the native hormone while significantly enhancing the half-life.
CNTO736 is a macromolecule containing two GLP-1 moieties, and our data demonstrate that its activity is similar to native GLP-1. CNTO736 binds to the GLP-1 receptor, induces cAMP production, and increases insulin secretion in a glucose-dependent manner. CNTO736 also reduces fasting and postprandial blood glucose and reduces A1C after chronic dosing.
One potential concern for any long-lived GLP-1 receptor agonist is that continual exposure to the peptide could result in receptor tachyphylaxis. However, continuous infusion of native GLP-1 in type 2 diabetic patients reduced blood glucose equally after 1 or 6 weeks of treatment, indicating that the receptor was present and accessible throughout treatment (23). A second study comparing a 16- vs. 24-h GLP-1 infusion in patients indicated that greater efficacy could be achieved with sustained 24-h coverage (38), with no evidence of tachyphylaxis or loss of activity. Finally, treatment with exendin-LAR has been reported to reduce A1C as much as 2% in the high-dose group (39). Since the half-life for exendin-LAR was reported to be 14–15 days, the data confirms that sustained exposure to a GLP-1 analogue has potential for profound efficacy. Diabetic mice dosed chronically with CNTO736 achieved a significant reduction in FBG, and an oral glucose tolerance test performed at the end of the study showed no evidence of tachyphylaxis. Consistent with a prolonged reduction in blood glucose levels, A1C was significantly lower in mice treated with CNTO736 compared with vehicle-treated db/db mice (7.2 ± 1.1 vs. 8.4 ± 1.1%, P < 0.05) (data not shown).
A second issue relates to the potential for an immune response in animals treated with CNTO736 that contains a human Fc. We anticipated that an immune response could attenuate the activity of CNTO736 in mouse models. Thus, although CNTO736 showed prolonged pharmacokinetics following a single dose in mice, animals were dosed daily to ensure continuous coverage in chronic experiments. Despite the likelihood of an anti-human Fc response, efficacy was maintained in mice dosed chronically with CNTO736.
A significant component of the observed efficacy in patients treated with byetta or liraglutide arises from a reduction in food intake and body weight (24,40–42). Our data demonstrate that peripheral dosing of CNTO736 decreases food intake and chronic dosing reduces body weight. It remains unclear whether peripherally or centrally located receptors mediate the GLP-1 effect on food intake, and we anticipated that the molecular weight of CNTO736 would preclude its passage across the blood-brain barrier. Immunohistochemistry was used to test the hypothesis directly, and CNTO736 was detected only in brain regions that are readily available to circulation (circumventricular organs). However, c-fos activation was observed in the amygdale. Therefore, GLP-1 receptors that influence food intake are likely located either in the periphery or in an area of the brain that is exposed to circulation. Our c-fos activation data are consistent with previous reports that neurons found in the area postrema are important for transmitting signals from the periphery to the central autonomic regulatory system (43).
A major adverse event associated with the incretin mimetics is nausea. Although the mechanism for nausea is not well understood, it has been speculated that a delay in gastric emptying may play a role. Alternatively, it is likely that there is a central component to the nausea. Experiments in which GLP-1 was dosed directly into various regions of rat brains followed by a conditioned taste aversion test suggest that receptors located in the central nucleus of the amygdala mediate visceral illness (44). A significant difference between CNTO736 and many GLP-1 receptor agonists evaluated in the clinic thus far is its size. Byetta and liraglutide are low–molecular weight peptides that are likely to cross the blood-brain barrier. Since the central nucleus of the amygdala is an area of the brain that is protected by the blood-brain barrier, CNTO736 is unable to directly activate receptors there following peripheral dosing. However, c-fos is activated in the amygdale following peripheral dosing of CNTO736. Peripheral dosing of albugon, another large molecule GLP-1 receptor agonist, also activated c-fos in many areas of the brain that are not likely to be accessible to large molecules, including the central nucleus of the amygdala (31). Thus, it is likely that there is a peripheral mechanism that allows for activation of receptors in central nervous system centers. Since c-fos signaling from albugon was less robust than that of exenatide, it is possible that central and peripheral mechanisms act in concert to mediate the complete pharmacology of GLP-1. It may be possible to separate the negative (i.e., nausea) events associated with GLP-1 therapeutics by minimizing localization in the central nervous system. Regardless of the predominant mechanism, a correlation between Cmax and nausea has been observed for GLP-1 receptor peptide agonists. A molecule with an extended and flattened pharmacokinetic profile may be expected to have fewer Cmax-related side effects. Taken together, we anticipate that the MIMETIBODY platform may enable the desired activities of GLP-1 with minimal adverse events.
In conclusion, the data presented suggest that our novel MIMETIBODY platform can be used to improve the properties of a bioactive peptide, rendering it more suitable for therapeutic use. CNTO736 is a potent GLP-1 analogue with potential to improve treatment of type 2 diabetes. The half-life of CNTO736 is substantially longer than native GLP-1, byetta, or liraglutide. It is tempting to speculate that sustaining superphysiological levels of a GLP-1 receptor agonist will translate into significant improvements in efficacy with less frequent dosing and the potential to inhibit disease progression. These data illustrate the potential for applying the MIMETIBODY platform to enhance the properties of agonist peptide therapeutics for metabolic diseases. More generally, identification of agonist antibodies or small molecule drugs for multitransmembrane receptors remains challenging, and the MIMETIBODY platform provides a technology to address this gap and enable peptide agonist drug development.
Acknowledgments
We thank the Diabetes Research Institute at the University of Miami for kindly providing human islets. In addition, we thank Cindy Duchala for preparation of rat islets. We thank Sreedevi Adhikarakunnathu, Heather Deutsch, Stacy Hudgins, Dorie Makropoulos, Christine McCauley, and Justin Sprenkle for expert technical assistance. We thank Eva Emmell, Michael Lark, Simon Blake, Peter Bugelski, and John O'Neil for critical advice and guidance.
Published ahead of print at http://diabetes.diabetesjournals.org on 21 April 2008.
The costs of publication of this article were defrayed in part by the payment of page charges. This article must therefore be hereby marked “advertisement” in accordance with 18 U.S.C. Section 1734 solely to indicate this fact.
REFERENCES
- 1.Kay BK, Kurakin AV, Hyde-DeRuyscher R: From peptides to drugs via phage display. DDT 3 :370 –378,1998 [Google Scholar]
- 2.Brown LR: Commercial challenges of protein drug delivery. Expert Opin Drug Deliv 2 :29 –42,2005 [DOI] [PubMed] [Google Scholar]
- 3.Kumar TR, Soppimath K, Nachaegarim SK: Novel delivery technologies for protein and peptide therapeutics. Curr Pharm Biotechnol 7 :261 –276,2006 [DOI] [PubMed] [Google Scholar]
- 4.Sato AK, Viswanathan M, Kent RB, Wood CR: Therapeutic peptides: technological advances driving peptides into development. Curr Opin Biotechnol 17 :638 –642,2006 [DOI] [PubMed] [Google Scholar]
- 5.Reichert JM, Rosensweig CJ, Faden LB, Dewitz MC: Monoclonal antibody successes in the clinic. Nat Biotechnol 23 :1073 –1078,2005 [DOI] [PubMed] [Google Scholar]
- 6.Carter P: Potent antibody therapeutics by design. Nat Rev Immunol 6 :343 –357,2006 [DOI] [PubMed] [Google Scholar]
- 7.Drucker DJ, Philippe J, Mojsov S, Chick WL, Habener JF: Glucagon-like peptide I stimulates insulin gene expression and increases cyclic AMP levels in a rat islet cell line. Proc Natl Acad Sci U S A 84 :3431 –3438,1987 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Orskov C, Holst JJ, Knuhtsen S, Baldissera FG, Poulsen SS, Nielsen OV: Glucagon-like peptides GLP-1 and GLP-2, predicted products of the glucagon gene, are secreted separately from pig small intestine but not pancreas. Endocrinology 119 :1467 –1475,1986 [DOI] [PubMed] [Google Scholar]
- 9.Mojsov S, Heinrich G, Wilson IB, Ravazzola M, Orci L, Habener JF: Preproglucagon gene expression in pancrea and intestine diversifies at the level of post-translational processing. J Biol Chem 261 :11880 –11884,1986 [PubMed] [Google Scholar]
- 10.Holst JJ, Orskov C, Vagn Nielsen O, Schwartz TW: Truncated glucagon-like peptide I, an insulin-releasing hormone from the distal gut. FEBS Lett 211 :169 –174,1987 [DOI] [PubMed] [Google Scholar]
- 11.Marguet D, Baggio L, Kobayashi T, Bernard AM, Pierres M, Nielsen PF, Ribel U, Watanabe T, Drucker DJ, Wagtmann N: Enhanced insulin secretion and improved glucose tolerance in mice lacking CD26. Proc Natl Acad Sci U S A 97 :6874 –6879,2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kieffer TJ, McIntosh CHS, Pederson RA: Degradation of glucose-dependent insulinotropic polypeptide and truncated glucagon-like peptide 1 in vitro and in vivo by dipeptidyl peptidase IV. Endocrinology 136 :3585 –3596,1995 [DOI] [PubMed] [Google Scholar]
- 13.Kreymann B, Williams G, Ghatei MA, Bloom SR: Glucagon-like peptide-1 7–36: a physiological incretin in man. Lancet II :1300 –1303,1987 [DOI] [PubMed] [Google Scholar]
- 14.Wettergren A, Schjoldager B, Mortensen PE, Myhre J, Christiansen J, Holst JJ: Truncated GLP-1 (proglucagon 78–107-amide) inhibits gastric and pancreatic functions in man. Dig Dis Sci 38 :665 –673,1993 [DOI] [PubMed] [Google Scholar]
- 15.Little TJ, Pilichiewicz AN, Russo A, Phillips L, Jones KL, Nauck MA, Wishart J, Horowitz M, Feinle-Bisset C: Effects of intravenous GLP-1 on gastric emptying and intragastric distribution in healthy subjects: relationships with postprandial glycemic and insulinemic responses. J Clin Endocrin Metab 91 :1916 –1923,2006 [DOI] [PubMed] [Google Scholar]
- 16.Perfetti R, Zhou J, Doyle ME, Egan JM: Glucagon-like peptide-1 induces cell proliferation and pancreatic-duodenum homeobox-1 expression and increases endocrine cell mass in the pancreas of old, glucose-intolerant rats. Endocrinology 141 :4600 –4605,2000 [DOI] [PubMed] [Google Scholar]
- 17.Edvell A, Lindstrom P: Initiation of increased pancreatic islet growth in young normoglycemic mice (umea +/?). Endocrinology 140 :778 –783,1999 [DOI] [PubMed] [Google Scholar]
- 18.Hardikar AA, Wang XY, Williams LJ, Kwok J, Wong R, Yao M, Tuch BE: Functional maturation of fetal porcine b-cells by glucagon-like peptide 1 and cholecystokinin. Endocrinology 143 :3505 –3514,2002 [DOI] [PubMed] [Google Scholar]
- 19.Li Y, Hansotia T, Yusta B, Ris F, Halban PA, Drucker DJ: Glucagon-like peptide-1 receptor signaling modulates b-cell apoptosis. J Biol Chem 278 :471 –478,2003 [DOI] [PubMed] [Google Scholar]
- 20.Farilla L, Hui H, Bertolotto C, Kang E, Bulotta A, Di Mario U, Perfetti R: Glucagon-like peptide-1 promotes islet cell growth and inhibits apoptosis in Zucker diabetic rats. Endocrinology 143 :4397 –4408,2002 [DOI] [PubMed] [Google Scholar]
- 21.Farilla L, Bulotta A, Hirshberg B, Calzi SL, Khoury N, Noushmehr H, Bertolotto C, DiMario U, Harlan DM, Perfetti R: Glucagon-like peptide 1 inhibits cell apoptosis and improves glucose responsiveness of freshly isolated human islets. Endocrinology 144 :5149 –5158,2003 [DOI] [PubMed] [Google Scholar]
- 22.Buteau J, El-Assaad W, Rhodes CJ, Rosenberg L, Joly E, Prentki M: Glucagon-like peptide-1 prevents beta cell glucolipotoxicity. Diabetologia 47 :806 –815,2004 [DOI] [PubMed] [Google Scholar]
- 23.Zander M, Madsbad S, Madsen JL, Holst JJ: Effect of 6-week course of glucagon-like peptide 1 on glycaemic control, insulin sensitivity, and b-cell function in type 2 diabetes: a parallel-group study. Lancet 359 :824 –830,2002 [DOI] [PubMed] [Google Scholar]
- 24.DeFronzo RA, Ratner RE, Han J, Kim DD, Fineman MS, Baron AD: Effects of exenatide (exendin-4) on glycemic control and weight over 30 weeks in metformin-treated patients with type 2 diabetes. Diabetes Care 28 :1092 –1100,2005 [DOI] [PubMed] [Google Scholar]
- 25.Bugelski PJ, Capocasale RJ, Makropoulos D, Marshall D, Fisher PW, Lu J, Achuthanandam R, Spinka-Doms T, Kwok D, Graden D, Volk A, Nesspor T, James IE, Huang C: CNTO 530: molecular pharmacology in human UT-7EPO cells and pharmacokinetcs and pharmacodynamics in mice. J Biotechnology 134 :171 –180,2008 [DOI] [PubMed] [Google Scholar]
- 26.O'Neil KT, Picha K: Human GLP-1 mimetibodies, compositions, methods and uses. Patent Publication: WO 2005097175A2
- 27.Ritzel U, Leonhardt U, Ottleben M, Ruhmann A, Eckart K, Spiess J, Ramadori G: A synthetic glucagon-like peptide-1 analog with improved plasma stability. J Endocrinol 158 :93 –102,1998 [DOI] [PubMed] [Google Scholar]
- 28.Xiao Q, Giguere J, Parisien M, Jeng W, St. Pierre SA, Brubaker PL, Wheeler MB: Biological activities of glucagon-like peptide-1 analogues in vitro and in vivo. Biochemistry 40 :2860 –2869,2001 [DOI] [PubMed] [Google Scholar]
- 29.Bottino R, Fernandez LA, Ricordi C, Lehmann R, Tsan M, Oliver R, Inverardi L: Transplantation of allogeneic islets of langerhans in the rat liver: effects of marophage depletion on graft survival and microenvironment activation. Diabetes 47 :316 –323,1998 [DOI] [PubMed] [Google Scholar]
- 30.Scrocchi LA, Brown TJ, Maclusky N, Brubaker PL, Auerbach AB, Joyner AL, Drucker DJ: Glucose intolerance but normal satiety in mice with a null mutation in the glucagon-like peptide 1 receptor gene. Nat Med 2 :1254 –1258,1996 [DOI] [PubMed] [Google Scholar]
- 31.Baggio LL, Huang Q, Brown TJ, Drucker, D. J: A recombinant glucagon-like peptide (GLP)-1 albumin protein (albugon) mimics peptidergic activation of GLP-1 receptor–dependent pathways coupled with satiety, gastrointestinal motility, and glucose homeostasis. Diabetes 53 :2492 –2500,2004 [DOI] [PubMed] [Google Scholar]
- 32.Holliger P, Hudson PJ: Engineered antibody fragments and the rise of single domains. Nat Biotechnol 23 :1126 –1136,2005 [DOI] [PubMed] [Google Scholar]
- 33.Glaesner W, Micanovic R, Tschang S: GLP-1 fusion proteins. Patent Publication: WO 200246227A2
- 34.Suzuki S, Kawai K, Ohashi S, Mukai H, Yamashita K: Comparison of the efffects of various C-terminal and N-terminal fragment peptides of glucagon-like peptide-1 on insulin and glucagon release from the isolated perfused rat pancreas. Endocrinology 125 :3109 –3114,1989 [DOI] [PubMed] [Google Scholar]
- 35.Tang-Christensen M, Larsen PJ, Goke R, Fink-Jensen A, Jessop DS, Moller M, Sheikh SP: Central administration of GLP-1 (7–36) amide inhibits food and water intake in rats. Am J Physiol 271 :R848 –R856,1996 [DOI] [PubMed] [Google Scholar]
- 36.Turton MD, O'Shea D, Gunn I, Beak SA, Edwards CMB, Meeran K, Choi SJ, Taylor GM, Heath MM, Lambert PD, Wilding JPH, Smith DH, Ghatei MA, Herbert J, Bloom SR: A role for glucagon-like petide-1 in the central regulation of feeding. Nature 379 :69 –72,1996 [DOI] [PubMed] [Google Scholar]
- 37.van Dijk G, Thiele TE, Seeley RJ, Woods SC, Bernstein IL: Glucagon-like peptide-1 and satiety. Nature 385 :214 ,1997 [DOI] [PubMed] [Google Scholar]
- 38.Larsen J, Hylleberg B, Ng K, Damsbo P: Glucagon-like peptide-1 infusion must be maintained for 24 h/day to obtain acceptable glycemia in type 2 diabetic patients who are poorly controlled on sulphonylurea treatment. Diabetes Care 24 :1416 –1421,2001 [DOI] [PubMed] [Google Scholar]
- 39.Kim D, MacConell L, Zhuang D, Kothare PA, Trautman M, Fíneman M, Taylor K: Effects of once-weekly dosing of a long-acting release formulation of exenatide on glucose control and body weight in subjects with type 2 diabetes. Diabetes Care 30 :1487 –1493,2007 [DOI] [PubMed] [Google Scholar]
- 40.Vilsboll T, Zdravkovic M, Le-Thi T, Krarup T, Schmitz O, Courreges J, Verhoeven R, Buganova I, Madsbad S: Liraglutide Significantly Improves Glycemic Control, and Lowers Body Weight without Risk of Either Major or Minor Hypoglycemic Episodes in Subjects with Type 2 Diabetes. Matschinsky FM, Banks P, Ed. Washington, DC, American Diabetes Association,2006. , p.A27 –A28
- 41.Buse JB, Henry RR, Han J, Kim DD, Fineman MS, Baron AD: Effects of exenatide (exendin-4) on glycemic control over 30 weeks in sulfonylurea-treated patients wtih type 2 diabetes. Diabetes Care 27 :2628 –2635,2004 [DOI] [PubMed] [Google Scholar]
- 42.Kendall DM, Riddle MC, Rosenstock J, Zhuang D, Kim DD, Fineman MS, Baron AD: Effects of exenatide (Exendin-4) on glycemic control over 30 weeks in patients with type 2 diabetes treated with metformin and a sulonylurea. Diabetes Care 28 :1083 –1091,2005 [DOI] [PubMed] [Google Scholar]
- 43.Yamamoto H, Kishi T, Lee CE, Choi BJ, Fang H, Hollenberg AN, Drucker DJ, Elmquist JK: Glucagon-like peptide-1-responsive catecholamine neurons in the area postrema link peripheral glucagon-like peptide-1 with central autonomic control sites. J Neuroscience 23 :2939 –2946,2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Kinzig KP, D'Alessio DA, Seeley RJ: The diverse roles of specific GLP-1 receptors in the control of food intake and the response to visceral illness. J Neuroscience 22 :10470 –10476,2002 [DOI] [PMC free article] [PubMed] [Google Scholar]