Abstract
OBJECTIVE—Weak major histocompatibility complex (MHC) binding of self-peptides has been proposed as a mechanism that may contribute to autoimmunity by allowing for escape of autoreactive T-cells from the thymus. We examined the relationship between the MHC-binding characteristics of a β-cell antigen epitope and T-cell autoreactivity in a model of autoimmune diabetes.
RESEARCH DESIGN AND METHODS—The binding of a proinsulin epitope, proinsulin-1(47–64) (PI-1[47–64]), to the MHC class II molecules I-Ag7 and I-Ak was measured using purified class II molecules. T-cell reactivity to the proinsulin epitope was examined in I-Ag7+ and I-Ak+ mice.
RESULTS—C-peptide epitopes bound very weakly to I-Ag7 molecules. However, C-peptide–reactive T-cells were induced after immunization in I-Ag7–bearing mice (NOD and B6.g7) but not in I-Ak–bearing mice (B10.BR and NOD.h4). T-cells reactive with the PI-1(47–64) peptide were found spontaneously in the peripancreatic lymph nodes of pre-diabetic NOD mice. These T-cells were activated by freshly isolated β-cells in the presence of antigen-presenting cells and caused diabetes when transferred into NOD.scid mice.
CONCLUSIONS—These data demonstrate an inverse relationship between self-peptide–MHC binding and T-cell autoreactivity for the PI-1(47–64) epitope in autoimmune diabetes.
Defective negative selection caused by weak interactions between self-peptides and major histocompatibility complex (MHC) molecules has been proposed as a mechanism that may contribute to autoimmunity. We have been interested in examining the relationship between peptide-MHC interactions and T-cell autoreactivity for disease-relevant antigens in autoimmune diabetes. In the case of the nonobese diabetic (NOD) mouse, the class II MHC molecule, I-Ag7, has been shown to be a weak peptide binder (1). While examining the CD4 T-cell response to peptides derived from the insulin β-chain in the NOD mouse, we were struck by its low binding affinity to the I-Ag7 class II MHC molecule (2). Many of the spontaneous T-cells that were identified reacted to a segment previously identified as a focus for spontaneous T-cell reactivity (3–6). Such a peptide apparently bound in two registers to I-Ag7, but binding was in the low micromolar range and showed a fast dissociation rate. Many of the amino acids in the peptide affected both binding and T-cell recognition, implying a very loose peptide-MHC complex (2).
The relationship of low affinity of a peptide to an MHC molecule and autoimmune reactivity has also been noted in the encephalitogenic peptide from myelin basic protein (7,8). This has led to the speculation that low-affinity peptides may not be conducive to the normal mechanisms in the thymus that control autoreactivity. A study by McNeil and Evavold noted that peptides with fast dissociation rates and poor binding to MHC molecules were ineffective at deleting thymocytes even though the same ligands were capable of eliciting proliferation of mature T-cells in the periphery (9). Similarly, in a tumor model system, low-affinity epitopes were shown to activate tumor-specific cytotoxic T-lymphocytes and could confer antitumor immunity on immunization (10). Lastly, a study by Roep and colleagues (11) that measured in vitro binding interactions of proinsulin epitopes to various HLA-DR molecules noted that DR alleles associated with protection from diabetes bound various proinsulin epitopes with a higher affinity when compared with DR molecules that predispose to type 1 diabetes.
To further characterize the relationship between peptide-MHC binding strength and immune reactivity, we have examined other regions of the proinsulin molecule targeted by CD4 T-cells. The insulin molecule is derived from the processing of the prohormone proinsulin and is secreted from the pancreatic β-cell with equimolar amounts of the connecting peptide C-peptide (Table 1) (12). We examined the proinsulin-1(47–64) (PI-1[47–64]) (SPGDLQTLALEVARQKRG) segment because reactivity to it had been found in NOD mice (5,13). These data extend our understanding of the role of proinsulin as an autoantigen in type 1 diabetes and demonstrate an inverse relationship between peptide-MHC affinity and T-cell autoreactivity against a defined β-cell antigen epitope.
TABLE 1.
Human and murine proinsulin sequences
| 1———————9—————————————23———-30 | |
| Human (b) | FVNQHLCGSHLVEALYLVCGERGFFYTPKT |
| Mouse I | FVKQHLCGPHLVEALYLVCGERGFFYTPKS |
| Mouse II | FVNQHLCGSHLVEALYLVCGERGFFYTPMS |
| 31————————————-47——49———————————61 | |
| Human (C) | RREAEDLQVGQVELGGGPGAGSLQPLALEGSLQKR |
| Mouse I | RREVEDPQVEQLELGGSP GDLQTLALEVARQKR |
| Mouse II | RREVEDPQVAQLELGGGPGAGDLQTLALEVAQQKR |
| 64————————————————-86 | |
| Human (a) | GIVEQCCTSICSLYQLENYCN |
| Mouse I | GIVDQCCTSICSLYQLENYCN |
| Mouse II | GIVDQCCTSICSLYQLENYCN |
The sequences of the human, mouse-1, and mouse-2 proinsulin molecules are shown. The amino acid residues in bold are cleaved off during the physiological processing of the prohormone, and C-peptide is secreted in equimolar amount with the mature insulin heterodimer. The amino acid residue numbering refers to the mouse proinsulin-1 sequence, and the PI-1(47–64) segment is underlined.
RESEARCH DESIGN AND METHODS
NOD, NOD.scid, NOD.h4, B6.g7, and B10.BR mice were purchased from The Jackson Laboratories (Bar Harbor, ME). All mice were housed and cared for in accordance with the guidelines of the Washington University Committee for the Humane Care of Laboratory Animals and with National Institutes of Health guidelines on laboratory animal welfare.
Peptides.
Synthetic peptides were purchased from Biosynthesis (Lewisville, TX). All peptides were >95% pure, confirmed by high-performance liquid chromatography and mass spectrometry analysis.
Peripancreatic lymph node assays.
Spleens, axillary and inguinal lymph nodes, and peripancreatic lymph nodes (PPLNs) were isolated from 10- to 16-week-old male and female NOD mice and dispersed into single-cell suspensions by passage through cell strainers. The splenocytes or lymph node cells (3–5 × 104/well) were subsequently cultured with irradiated NOD splenocytes (5 × 105/well) and 1–5 μmol/l peptide for 7 days in round-bottom 96-well plates in a final volume of 200 μl. On day 7, the cultures were re-stimulated with fresh irradiated NOD splenocytes (5 × 105/well), 25 units/ml interleukin (IL)-2, and 1–5 μmol/l peptide. T-cell–positive wells were tested days 14–17 with antigen-presenting cells (APCs) and 10 μmol/l peptide. Only data from cells with >200 counts per minute were considered, the stimulation index was calculated by dividing values for APCs plus peptide by APCs alone. The data presented in Fig. 2A were pooled from seven independent experiments. All tissue culturing was done in Dulbecco's modified Eagle's medium (DMEM) supplemented with 10% FCS (DMEM 10% FCS).
FIG. 2.
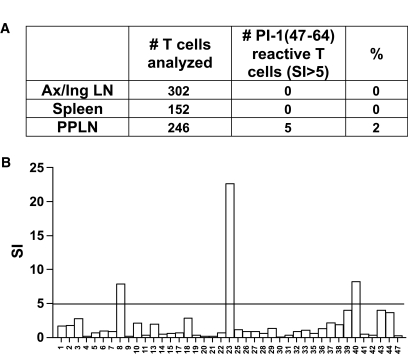
PPLNs contain T-cells reactive with PI-1(47–64). A: T-cells from the PPLNs of NOD mice from 10–16 weeks of age contain PI-1(47–64)–reactive T-cells. Five of 246 expanded T-cell lines from the PPLNs reacted with the SPGDLQTLALEVARQKRG peptide with a stimulation index (SI) >5. In contrast, 0 of 454 T-cell lines expanded from the spleen or axillary and inguinal lymph nodes were stimulated by the PI-1(47–64) peptide. B: Data from one representative experiment in which 41 T-cell lines from the PPLNs were tested for reactivity with the PI-1(47–64) peptide.
Primary T-cell lines.
The anti–C-peptide (ACP) primary T-cell lines were generated by immunization of 8- to 10-week-old NOD mice with 10 nmol PI-1(47–64) in complete Freund's adjuvant (CFA). Immunized lymph nodes were harvested at day 7 and cultured at 5 × 105 cells/well in DMEM with 10% FCS and 1 μmol/l peptide. On day 7, and at 7- to 10-day intervals thereafter, the T-cells (1–2 × 103–4/w) were restimulated with fresh irradiated NOD splenocytes (5 × 105 cells/well), IL-2 (25 units/ml), and peptide (1 μmol/l). Hybridomas were generated from these primary lines by fusion to the BW5147 α-β-thymoma partner cell line following standard techniques (14).
T-cell assays.
Primary T-cell proliferation assays were performed by incubation of T-cells (2 × 104/well) with irradiated NOD splenocytes (5 × 105/well) in the presence of peptide or dispersed mouse β-cells. Primary T-cell proliferation assays were pulsed with thymidine (1 μCi/well) on day 2 and harvested day 3. T-cell hybridoma assays were performed with NOD splenocytes or the I-Ag7–expressing cell line, C3G7 (2.5 × 104/well), as APCs and supernatants were collected at 24 h for IL-2 quantitation by cytotoxic T-lymphocyte line assay. Fixation of the C3G7 cell line was performed by incubation in 1% paraformaldehyde for 15 min followed by incubation in 0.2 mol/l dl-lysine for 10 min and then washing three times in DMEM 10% FCS.
Islet isolation.
Islets of Langerhans were isolated from mice of various ages and dispersed using standard techniques (15). The digestion of the pancreatic tissue was performed with type XI collagenase (Sigma, St. Louis, MO) without DNase.
Enzyme-linked immunosorbent spot-forming cell analysis.
Mice were immunized with 10 nmol PI-1(47–64) peptide in CFA and lymph nodes were examined on day 7 by enzyme-linked immunosorbent spot-forming cell (ELISPOT) analysis for IL-2+ cells. Lymph node cells (1 × 106/well) were plated in triplicate on anti–IL-2 capture antibody–coated filter plates in the presence of 10 μmol/l antigen and developed 24 h later with anti–IL-2 detection antibodies (BD Biosciences, San Jose, CA). Plates were read by C.T.L. Cellular Technology and analyzed with ImmunoSpot 3.2 software.
Peptide-binding assays.
Soluble I-Ag7 or I-Ak was produced using the recombinant baculovirus system as previously described (16). Peptide binding assays were done under acidic (pH 5.5) conditions. Briefly, 0.5–1 μg I-Ag7 or I-Ak/class II–associated invariant chain peptide was treated with 0.1 unit thrombin to cleave both the zipper tails and peptide linker (Novagen, Madison, WI) and simultaneously incubated with 0.125 pmol I125-radiolabeled mimotope reference peptide (GKKVATTVHAGYG) and increasing doses of unlabeled peptides in 200 mmol/l Tris(2-carboxyethyl) phosphine hydrochloride, 20 mmol/l MES, and 150 mmol/l sodium chloride. Binding reactions were incubated overnight at 25°C in 30-μl volumes. Complexes were purified from free peptide by gel filtration Bio-spin columns (Bio-Rad). The percentage of bound peptide was evaluated by gamma counting. Usually, ∼25–35% of input peptide was bound, whereas <0.5% of peptides nonspecifically passed through the Bio-spin columns. The half-maximal inhibitory concentration (IC50) value is very close to the binding equilibrium constant. The IC50 values shown in Table 2 are averages of three to five independent binding experiments.
TABLE 2.
Binding to I-Ag7
| Peptide | Sequence | Binding |
|
|---|---|---|---|
| I-Ag7 | I-Ak | ||
| MIME | GKKVATTVHAGYG | 1 | — |
| PI-1(47–64) | SPGDLQTLALEVARQKRG | 98.9 | 4.8 |
| PI-2(49–66) | GAGDLQTLALEVAQQKRG | 135.9 | 2.1 |
| PI-1(49–61) | GDLQTLALEVARQ | 20.0 | — |
| INS-B(9–23) | SHLVEALYLVCGERG | 3 | — |
This panel shows the binding analysis of synthetic peptides to soluble I-Ag7 and I-Ak. Binding is expressed by IC50 values (μmol/l). The mime peptide is a synthetic reference peptide that has been used in I-Ag7 binding assays (16). The binding of mouse insulin-2 B:(9–23) (3 μmol/l) peptide is presented as a point of reference. Binding studies were performed at pH 5.5 as described in research design and methods.
Biological dissociation rates.
The C3G7 APC line was incubated with peptide (10 μmol/l) for 2 h and washed. ACP T-cells (2 × 104/well) were added to the APC-containing wells (2.5 × 104/well) cultures at time zero and at 4 and 8 h after washing. The supernatants were collected at 24 h, and IL-2 was measured by cytotoxic T-lymphocyte line assay.
Adoptive T-cell transfer.
Two of the three primary ACP T-cell lines were expanded in vitro and transferred into 8- to 12-week-old NOD.scid recipients by tail vein injection. Diabetes was defined by the measurement of blood sugars >250 mg/dl on two separate occasions. All recipients were followed for 30 weeks or up to disease onset.
RESULTS
The PI-1(47–64) peptide bound weakly to I-Ag7.
Binding assays were done with soluble I-Ag7 and I-Ak and synthetic peptides. The IC50 value indicates the concentration of unlabeled peptide required to affect binding of a reference peptide to the class II molecules: the higher the value, the weaker the binding of the peptide to the MHC. The PI-1(47–64) and PI-2(49–66) peptides bound poorly to I-Ag7, with IC50 values of 98.9 and 135.9 μmol/l, respectively (Table 2). In contrast, these peptides bound more strongly to I-Ak, with IC50 values of 4.8 and 2.1 μmol/l, respectively. The PI-1(49–61) peptide bound slightly better to I-Ag7, with an IC50 of 20 μmol/l, perhaps due to the loss of the positively charged P62Lys, which is likely disfavored at the carboxy-end of the peptide-binding groove of I-Ag7. As a point of reference, the mouse insulin-2 β-chain (9–23) peptide bound to I-Ag7 with an IC50 of 3 μmol/l.
Reactivity to PI-1(47–64) is I-Ag7 dependent.
The role played by the MHC class II molecule, I-Ag7, and background genes in autoreactivity to C-peptide was examined by characterizing the T-cell responses in NOD, NOD.h4, B6.g7, and B10.BR mice. Mice were immunized with the PI-1(47–64) peptide, and lymph nodes were examined on day 7 by ELISPOT analysis for IL-2+ cells. Both I-Ag7–containing strains, NOD and B6.g7, had peptide-reactive T-cells (Fig. 1A and C), compared with the I-Ak+ strains that did not (Fig. 1B and D). The frequency of PI-1(47–64)–reactive cells detected under these conditions was low: ∼1/30,000 in the NOD mice and 1/50,000 in the B6.g7 mice. However, PI-1(47–64)–specific T-cells were not detected in the NOD.h4 and B10.BR mice. The T-cell reactivity against PI-1(47–64) present in the NOD and B6.g7 mice was clearly I-Ag7 restricted as evidenced by inhibition with MHC class II–blocking antibody, AG.2.42.7 (17). These data demonstrate that I-Ag7 alone is necessary and sufficient for the generation of PI-1(47–64) T-cells independent of other background genes.
FIG. 1.
The ACP T-cell repertoire in NOD mice is I-Ag7 dependent. Both I-Ag7–containing strains, NOD and B6.g7, had peptide-reactive T-cells (A and C), compared with the I-Ak+ strains, NOD.h4 and B10.BR, that did not (B and D). The frequency of PI-1(47–64)–reactive cells detected under these conditions was ∼1/30,000 in the NOD mice and 1/50,000 in the B6.g7 mice. However, PI-1(47–64)–specific T-cells were not detected in the NOD.h4 and B10.BR mice. The T-cell response to PI-1(47–64) in NOD and B6.g7 mice was MHC class II restricted as evidenced by inhibition with the MHC class II–blocking antibody, AG.2.42.7. Data presented are representative of three independent experiments.
PPLNs harbor C-peptide–reactive T-cells.
Given the central importance of the PPLNs in the priming of diabetogenic T-cells, we began by examining the PPLNs of pre-diabetic mice for the presence of T-cells specific for the PI-1(47–64) peptide. Like others (5,18), we did not detect reactivity to proinsulin peptides in primary proliferation assays with PPLNs or spleens from 10- to 16-week-old NOD mice (data not shown). Given this difficulty in detecting primary T-cell responses, we expanded PPLN T-cells, in addition to T-cells from the spleen and peripheral lymph nodes, for 2 weeks in vitro with the PI-1(47–64) peptide, and then tested all T-cells in the expanded cultures for peptide reactivity. T-cells specific for the PI-1(47–64) peptide were identified in the PPLNs from 10- to 16-week-old mice but were not found in the spleen or axillary and inguinal lymph nodes (Fig. 2A and B). Five of 246 expanded T-cell lines from the PPLNs reacted with the PI-1(47–64) peptide (stimulation index >5). In contrast, no PI-1(47–64)–reactive T-cells were detected from the 454 T-cell lines expanded from the spleen or axillary and inguinal lymph nodes. The presence of C-peptide–reactive T-cells in the PPLNs was in agreement with previous data that indicated these nodes as the site for priming of diabetogenic T-cells (19–21). Moreover, this finding also suggested a possible role for these T-cells in the early pathogenesis of the disease. To further characterize the biology of C-peptide–reactive T-cells, we first generated T-cell lines by immunization of young NOD mice.
NOD mice lack tolerance to the PI-1(47–64) self-peptide.
Mice were immunized with PI-1(47–64) peptide in CFA, and several primary CD4+ T-cell lines were generated, including the ACP 2 line (Fig. 3A). All lines demonstrated similar reactivity in vitro; they were maximally stimulated by the PI(47–64) peptide from proinsulin-1 and also reactive with the PI(49–66) peptide from proinsulin-2. The T-cell lines also recognized the PI-1(49–61) and the PI-2(51–63) segments, which differ only by one residue (Arg for PI-1 at position 60 and Gln for PI-2 at position 62). The T-cell lines were stimulated by the full-length C-peptides from proinsulin 1 and 2, although less than they were by the PI-1(47–64) peptide.
FIG. 3.
Primary anti–PI-1(47–64) T-cell lines are reactive with β-cells. A: Three primary anti–PI-1(47–64) T-cell lines were generated by immunization of young NOD mice. One such line, ACP no. 2, recognized the PI-1(47–64) peptide in addition to the PI-1(49–61) segment and full-length C-peptide-1. The ACP no. 2 T-cell line recognized peptides from the PI-2 sequence, although not as well as PI-1. B: The ACP no. 2 T-cell line responded to freshly isolated β-cells from 4- to 6-week-old NOD mice. In both peptide and β-cell assays, irradiated NOD splenocytes were used as APCs and cultures were pulsed with labeled thymidine on day 2 and harvested on day 3. C: ACP no. 1 reacted to dispersed islets from 5-month-old NOD mice in the absence of exogenous APCs.
To determine whether the ACP T-cell lines were reactive with the naturally processed and presented forms of the proinsulin epitope, T-cells were tested for reactivity with freshly isolated mouse β-cells. All three primary ACP lines, including ACP 2, reacted to dispersed mouse β-cells from young NOD mice (4–6 weeks of age) and β-cells from other strains such as 129/Svj (Fig. 3B). Furthermore, the ACP T-cells reacted to dispersed islets isolated from 5-month-old NOD mice, demonstrating that APCs from the islet infiltrate present the C-peptide epitope (Fig. 3C).
Minimal epitope mapping and antigen processing requirements for C-peptide–reactive T-cells.
Analysis of ACP T-cell hybridomas showed heterogeneity in their reactivity to truncated peptides (Fig. 4 A; Table 3). The likely minimal T-cell epitope spanned the PI-1(51–61) sequence (LQTLALEVARQ) because loss of the P51Leu resulted in a noticeable decrease in T-cell activation for all hybridomas examined (Fig. 4A; Table 3). Some T-cells, such as ACP2.1, responded well to the PI-1(49–60) peptide lacking the P61Gln, whereas others did not (see ACP1.28), indicating variable requirements for carboxy-terminal residues. Identification of the nonamer canonical core within the PI-1(51–61) segment could not be determined by T-cell reactivity, and all T-cells tested (0 of 8) with the three possible nonamers spanning the PI-1(51–61) peptide were negative (51–59, LQTLALEVA; 52–60, QTLALEVAR; and 53–61, TLALEVARQ). These results may indicate a need for amino- or carboxy-flanking residues that serve as T-cell receptor contacts, as was seen with the β-chain (9–23) epitope (2), loss of MHC binding affinity with the truncated peptides, or both.
FIG. 4.
ACP T-cells respond to PI-1(49–61), and full-length C-peptide presented by fixed APCs and the peptide-MHC (pMHC) complexes disassociate rapidly. A: The ACP1.28 T-cell responds well to PI-1(49–61):GDLQTLALEVARQ; however, reactivity was abolished with the loss of the P51Leu or the P61Gln. The ACP2.1 T-cell, in contrast, appeared to tolerate truncations of the flanks and recognized PI-1(49–60):GDLQTLALEVAR rather well and responded weakly to both PI-1(52–61):QTLALEVARQ and PI-1(49–59):GDLQTLALEVA. B: Both ACP1.28 and ACP2.1 responded strongly to fixed APCs pulsed with peptide, indicating that further processing of the peptide was not required. This was true for the PI-1(47–64) and PI-1(49–61) epitopes and the full-length C-peptide-1 molecule (peptide concentration 30 μmol/l; experiments performed in triplicate). C: The stability of the pMHC complexes formed from PI-1(47–64) or C-peptide-1 was examined by adding T-cells to antigen-pulsed and washed APCs over an 8-h time course. The percent maximal response was determined by dividing the T-cell response at T4 or T8, in counts per minute by the value achieved when the T-cells were added at T0. APCs pulsed with either PI-1(47–64) or C-peptide-1 lost 50% of their ability to maximally stimulate T-cells in 3 h, indicating that the pMHC complexes disassociated rapidly on the surface of the APCs.
TABLE 3.
ACP T-cell half-maximal stimulatory concentration values
| 1.23 | 1.24 | 1.28 | 1.56 | 2.1 | 2.55 | 2.78 | 2.87 | |
|---|---|---|---|---|---|---|---|---|
| SPGDLQTLALEVARQKRG | 0.3 | 0.13 | 0.06 | 0.25 | 0.1 | 0.025 | 0.03 | 0.07 |
| GDLQTLALEVARQ | — | >30 | 0.9 | 3.5 | 2 | 1.2 | 1.5 | 6 |
| DLQTLALEVARQ | — | >30 | 4 | 6 | 2.5 | 1.6 | 2.5 | 6 |
| LQTLALEVARQ | — | >30 | 4 | 15 | 3 | 2.1 | 5 | 6 |
| QTLALEVARQ | — | — | — | — | >30 | 30 | >30 | >30 |
| GDLQTLALEVAR | — | — | >30 | 15 | 2 | — | — | — |
| GDLQTLALEVA | — | — | — | — | >30 | — | — | — |
| GDLQTLALEVAQQ | — | — | — | 0.6 | 0.5 | — | — | — |
Values shown are concentrations of peptide required to half-maximally stimulate a given T-cell (μmol/l; —, none detected). Values are averages of two to five independent experiments.
To assess the requirements for antigen processing of the C-peptide T-cell epitopes, T-cells were tested for reactivity with synthetic peptides presented by fixed APCs. We found that ACP T-cell hybridomas were stimulated by fixed APCs presenting the PI-1(47–64), PI-1(49–61), and full-length C-peptide (Fig. 4B), indicating that all three peptides, even the full-length C-peptide-1 29-mer, could form recognizable complexes without the need for further processing.
We next examined the stability of the peptide-MHC complexes formed by PI-1(47–64) and I-Ag7 by functional experiments in which T-cells were applied to antigen-pulsed and washed APCs over an 8-h time course (Fig. 4C). The antigen-pulsed APCs quickly lost their ability to stimulate C-peptide–reactive T-cells. The T-cell response, for both PI-1(47–64)–and C-peptide-1–pulsed APCs, was reduced to 50% of its maximal value in 3 h, indicating that the C-peptide–MHC complexes disassociated rapidly.
C-peptide–reactive T-cells induce diabetes.
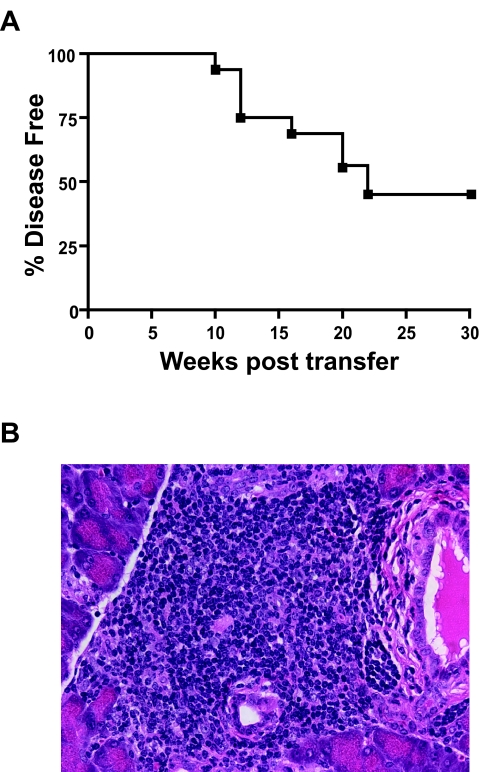
The pathogenicity of the ACP primary T-cell lines was assessed by adoptive transfer into NOD.scid recipient mice. Three separate transfer experiments were performed: group 1 (n = 8) received 9.4 × 106 ACP no. 1 T-cells; group 2 (n = 4) received 17 × 106 ACP no. 2 T-cells; and group 3 (n = 4) received three infusions of ACP nos. 1 and 2 T-cells. The incidence of diabetes was 50% for all groups; group 1 (4 of 8) by 20 weeks after transfer, group 2 (2 of 4) at 10 and 20 weeks after transfer, and group 3 (2 of 4) at 20 and 22 weeks after transfer (Fig. 5A). The induction of diabetes in 8/16 NOD.scid mice, which never naturally develop diabetes, indicated that the ACP T-cells were pathogenic in the adoptive transfer model. Although the kinetics of disease induction in these experiments is less than some observed with activated T-cells from T-cell receptor transgenic mice, e.g., BDC2.5 the incidence is similar to the spontaneous incidence seen in wild-type NOD mice. Furthermore, all recipient mice, including those that were not overtly hyperglycemic at 30 weeks, had severe destructive insulitis and loss of β-cell mass (Fig. 5B).
FIG. 5.
ACP T-cells transferred disease into recipient NOD.scid mice and caused severe insulitis. A: Adoptive transfer of ACP T-cells into NOD.scid recipient mice induced diabetes in 50% (8 of 16) by 22 weeks after transfer. See research design and methods for details. B: Histology of islets from all recipient mice, diabetic and normoglycemic, showed severe, destructive insulitis. The islet histology shown in this figure was taken from a nondiabetic recipient mouse at 30 weeks after transfer.
DISCUSSION
The main findings from these studies are 1) binding of the PI-1(47–64) and PI-1(49–61) peptides to I-Ag7 is incredibly poor, and the peptide-MHC complexes are short-lived; 2) I-Ag7 plays a central role in the generation of the ACP T-cell repertoire, in that autoreactivity was found to strains bearing I-Ag7 but not I-Ak molecules; 3) C-peptide–reactive T-cells are found in the PPLNs of pre-diabetic NOD mice; 4) ACP T-cells are reactive with mouse β-cells and respond to full-length C-peptide presented by live and fixed APCs; and 5) despite this weak peptide-MHC interaction, C-peptide–reactive T-cells are capable of causing diabetes in an adoptive transfer model.
Our data confirm and extend the observations of other groups that have identified the C-peptide region of proinsulin as a CD4+ T-cell target in the NOD mouse (5,13,22,23). The recovery of C-peptide–reactive T-cells from the PPLNs and not from other locations suggests that these T-cells may encounter their antigen in this critically important site of diabetogenic T-cell priming (19–21,24). Intra-islet APCs, most likely dendritic cells, must encounter large amounts of C-peptide in the islets and may traffic to the PPLNs, where they could prime and activate naïve C-peptide–reactive T-cells. Alternatively, lymphatic drainage of the pancreas might deliver high concentrations of C-peptide to the PPLNs, allowing uptake and presentation by nodal dwelling APCs. The islets readily provided antigen to APCs (Fig. 3B), and APCs recovered from insulitic islets stimulated the T-cells (Fig. 3C). In either case, it is not difficult to imagine a scenario in which PPLNs are exposed to large amounts of C-peptide, which would be required for activation of the T-cells by these very weak MHC-binding peptides. Although C-peptide circulates, its concentration in blood (2–5 nmol/l) would not be sufficient for either activation or regulation in APCs at peripheral sites.
Why are C-peptide–reactive T-cells not deleted in the thymus? Weak MHC-binding peptides may well be the best at fostering autoimmunity, in great part by avoiding negative selection in the thymus or by failure of regulatory mechanisms, although other factors are likely to play a role. The PI-1(47–64) epitope represents a striking example of a very poor binding MHC ligand, IC50 values ∼100 μmol/l, that gives rise to T-cells capable of causing diabetes in adoptive transfer experiments. The relationship between poor peptide-MHC binding and the fostering of an autoimmune, pathogenic T-cell repertoire has been suggested in studies on experimental allergic encephalomyelitis (7,8) and in our recent work on the insulin β-chain (9–23) epitope (2) and in other model systems (9–11).
The obligatory role for the diabetogenic I-Ag7 molecule in eliciting C-peptide–reactive CD4 T-cells remains a key issue. Our results with the four mouse strains NOD (I-Ag7+), NOD.h4 (I-Ak+), B6.g7 (I-Ag7+), and B10.BR (I-Ak+) demonstrated that expression of I-Ag7, independent of the genetic background, was sufficient to generate C-peptide–reactive CD4 T-cells (Fig. 1). This is in agreement with previous data from Kanagawa et al. (25) and Ridgway et al. (26), indicating that the expression of I-Ag7 in different strains of mice allowed escape of autoreactive T-cells. A more recent study from Stratmann et al. (27) used MHC tetramers to trace islet-reactive CD4+ T-cells in I-Ag7–expressing mice of different genetic backgrounds. Here again, the expression of I-Ag7 in different strains selected for tetramer-reactive CD4+ T-cells. The NOD strain has also been shown to be susceptible to other autoimmune diseases (28–31). Taken together, these data point to a central role for I-Ag7 in selection (or escape) of autoreactive T-cells. We believe that the explanation lies in the structural features of I-Ag7, characterized by an overall low peptide-binding affinity and instability (32), in addition to other susceptibility genes. These findings do not lessen the importance of other factors in NOD mice that play a role in the loss of self tolerance (33–35). The fact that B6.g7 mice do not develop diabetes highlights the need of both MHC- and non-MHC–encoded susceptibility genes.
A second important factor in the lack of T-cell tolerance may be specific to the insulin molecule. It may be that the peptide-MHC complexes formed in the thymus do not recapitulate the complexes that appear in the periphery. The deleting complexes are presumably formed by the traditional processing and presentation of proinsulin peptides in thymic medullary epithelial cells. Here, peptides are selected by the biological and physical forces that govern peptide presentation by I-Ag7 molecules in thymic cells and are not the same as events that might occur in the periphery. Although proinsulin is clearly expressed in the thymus (36,37), perhaps largely under the control of the autoimmune regulator transcription factor (38), there is no evidence of the expression of the prohormone processing machinery, such as the prohormone convertases 1 and 2, which would be required to generate the actual C-peptide fragment that is secreted by the β-cell. The absence of tolerance to C-peptide, as it is presented in the pancreas, may be explained, in a sense, by the lack of a secretory apparatus in the thymus.
The data presented here extend our understanding of the role of proinsulin as an autoantigen and again seem to illustrate an inverse relationship between weak peptide-MHC binding and T-cell autoimmune pathogenicity. The degree to which C-peptide–reactive T-cells contribute to disease pathogenesis in the NOD mouse cannot be determined with certainty. The T-cells were pathogenic in circumstances of adoptive transfer, and they represent one set of the many proinsulin-reactive T-cells that seem to play a key role in the development of autoimmune diabetes. The identification of T-cell reactivity against C-peptide epitopes in type 1 diabetic patients suggests that C-peptide might also be an important autoantigen in the human disease (39–42).
Acknowledgments
This work was supported by grants from the Barnes-Jewish Hospital Foundation, the National Institutes of Health (DK-067199 and DK-020279), and the Kilo Diabetes and Vascular Research Foundation.
We thank Shirley Petzold for technical assistance.
Published ahead of print at http://diabetes.diabetesjournals.org on 8 April 2008.
The costs of publication of this article were defrayed in part by the payment of page charges. This article must therefore be hereby marked “advertisement” in accordance with 18 U.S.C. Section 1734 solely to indicate this fact.
See accompanying commentary, p. 1788.
REFERENCES
- 1.Suri A, Levisetti MG, Unanue ER: Do the peptide-binding properties of diabetogenic class II molecules explain autoreactivity? Curr Opin Immunol 20 :105 –110,2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Levisetti MG, Suri A, Petzold SJ, Unanue ER: The insulin-specific T cells of nonobese diabetic mice recognize a weak MHC-binding segment in more than one form. J Immunol 178 :6051 –6057,2007 [DOI] [PubMed] [Google Scholar]
- 3.Daniel D, Gill RG, Schloot N, Wegmann D: Epitope specificity, cytokine production profile and diabetogenic activity of insulin-specific T cell clones isolated from NOD mice. Eur J Immunol 25 :1056 –1062,1995 [DOI] [PubMed] [Google Scholar]
- 4.Abiru N, Wegmann D, Kawasaki E, Gottlieb P, Simone E, Eisenbarth GS: Dual overlapping peptides recognized by insulin peptide B:9–23 T cell receptor AV13S3 T cell clones of the NOD mouse. J Autoimmun 14 :231 –237,2000 [DOI] [PubMed] [Google Scholar]
- 5.Halbout P, Briand JP, Becourt C, Muller S, Boitard C: T cell response to preproinsulin I and II in the nonobese diabetic mouse. J Immunol 169 :2436 –2443,2002 [DOI] [PubMed] [Google Scholar]
- 6.Nakayama M, Abiru N, Moriyama H, Babaya N, Liu E, Miao D, Yu L, Wegmann DR, Hutton JC, Elliott JF, Eisenbarth GS: Prime role for an insulin epitope in the development of type 1 diabetes in NOD mice. Nature 435 :220 –223,2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Fairchild PJ, Wildgoose R, Atherton E, Webb S, Wraith DC: An autoantigenic T cell epitope forms unstable complexes with class II MHC: a novel route for escape from tolerance induction. Int Immunol 5 :1151 –1158,1993 [DOI] [PubMed] [Google Scholar]
- 8.Liu GY, Fairchild PJ, Smith RM, Prowle JR, Kioussis D, Wraith DC: Low avidity recognition of self-antigen by T cells permits escape from central tolerance. Immunity 3 :407 –415,1995 [DOI] [PubMed] [Google Scholar]
- 9.McNeil LK, Evavold BD: Dissociation of peripheral T cell responses from thymocyte negative selection by weak agonists supports a spare receptor model of T cell activation. Proc Natl Acad Sci U S A 99 :4520 –4525,2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Gross DA, Graff-Dubois S, Opolon P, Cornet S, Alves P, Bennaceur-Griscelli A, Faure O, Guillaume P, Firat H, Chouaib S, Lemonnier FA, Davoust J, Miconnet I, Vonderheide RH, Kosmatopoulos K: High vaccination efficiency of low-affinity epitopes in antitumor immunotherapy. J Clin Invest 113 :425 –433,2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Geluk A, van Meijgaarden KE, Schloot NC, Drijfhout JW, Ottenhoff TH, Roep BO: HLA-DR binding analysis of peptides from islet antigens in IDDM. Diabetes 47 :1594 –1601,1998 [DOI] [PubMed] [Google Scholar]
- 12.Rubenstein AH, Clark JL, Melani F, Steiner DF: Secretion of proinsulin C-peptide by pancreatic beta cells and its circulation in blood. Nature 224 :697 –699,1969 [Google Scholar]
- 13.Jaeckel E, Lipes MA, von Boehmer H: Recessive tolerance to preproinsulin 2 reduces but does not abolish type 1 diabetes. Nat Immunol 5 :1028 –1035,2004 [DOI] [PubMed] [Google Scholar]
- 14.Kappler JW, Skidmore B, White J, Marrack P: Antigen-inducible, H-2-restricted, interleukin-2-producing T cell hybridomas: lack of independent antigen and H-2 recognition. J Exp Med 153 :1198 –1214,1981 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lacy PE, Kostianovsky M: Method for the isolation of intact islets of Langerhans from the rat pancreas. Diabetes 16 :35 –39,1967 [DOI] [PubMed] [Google Scholar]
- 16.Latek RR, Suri A, Petzold SJ, Nelson CA, Kanagawa O, Unanue ER, Fremont DH: Structural basis of peptide binding and presentation by the type I diabetes-associated MHC class II molecule of NOD mice. Immunity 12 :699 –710,2000 [DOI] [PubMed] [Google Scholar]
- 17.Suri A, Vidavsky I, van der Drift K, Kanagawa O, Gross ML, Unanue ER: In APCs, the autologous peptides selected by the diabetogenic I-Ag7 molecule are unique and determined by the amino acid changes in the P9 pocket. J Immunol 168 :1235 –1243,2002 [DOI] [PubMed] [Google Scholar]
- 18.Hurtenbach U, Maurer C: Type I diabetes in NOD mice is not associated with insulin-specific, autoreactive T cells. J Autoimmun 2 :151 –161,1989 [DOI] [PubMed] [Google Scholar]
- 19.Hoglund P, Mintern J, Waltzinger C, Heath W, Benoist C, Mathis D: Initiation of autoimmune diabetes by developmentally regulated presentation of islet cell antigens in the pancreatic lymph nodes. J Exp Med 189 :331 –339,1999 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Gagnerault MC, Luan JJ, Lotton C, Lepault F: Pancreatic lymph nodes are required for priming of beta cell reactive T cells in NOD mice. J Exp Med 196 :369 –377,2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Levisetti MG, Suri A, Frederick K, Unanue ER: Absence of lymph nodes in NOD mice treated with lymphotoxin-beta receptor immunoglobulin protects from diabetes. Diabetes 53 :3115 –3119,2004 [DOI] [PubMed] [Google Scholar]
- 22.Chen W, Bergerot I, Elliott JF, Harrison LC, Abiru N, Eisenbarth GS, Delovitch TL: Evidence that a peptide spanning the B-C junction of proinsulin is an early Autoantigen epitope in the pathogenesis of type 1 diabetes. J Immunol 167 :4926 –4935,2001 [DOI] [PubMed] [Google Scholar]
- 23.Martinez NR, Augstein P, Moustakas AK, Papadopoulos GK, Gregori S, Adorini L, Jackson DC, Harrison LC: Disabling an integral CTL epitope allows suppression of autoimmune diabetes by intranasal proinsulin peptide. J Clin Invest 111 :1365 –1371,2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Sarukhan A, Lechner O, von Boehmer H: Autoimmune insulitis and diabetes in the absence of antigen-specific contact between T cells and islet beta-cells. Eur J Immunol 29 :3410 –3416,1999 [DOI] [PubMed] [Google Scholar]
- 25.Kanagawa O, Martin SM, Vaupel BA, Carrasco-Marin E, Unanue ER: Autoreactivity of T cells from nonobese diabetic mice: an I-Ag7-dependent reaction. Proc Natl Acad Sci U S A 95 :1721 –1724,1998 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ridgway WM, Ito H, Fasso M, Yu C, Fathman CG: Analysis of the role of variation of major histocompatibility complex class II expression on nonobese diabetic (NOD) peripheral T cell response. J Exp Med 188 :2267 –2275,1998 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Stratmann T, Martin-Orozco N, Mallet-Designe V, Poirot L, McGavern D, Losyev G, Dobbs CM, Oldstone MB, Yoshida K, Kikutani H, Mathis D, Benoist C, Haskins K, Teyton L: Susceptible MHC alleles, not background genes, select an autoimmune T cell reactivity. J Clin Invest 112 :902 –914,2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Goillot E, Mutin M, Touraine JL: Sialadenitis in nonobese diabetic mice: transfer into syngeneic healthy neonates by splenic T lymphocytes. Clin Immunol Immunopathol 59 :462 –473,1991 [DOI] [PubMed] [Google Scholar]
- 29.Many MC, Maniratunga S, Denef JF: The non-obese diabetic (NOD) mouse: an animal model for autoimmune thyroiditis. Exp Clin Endocrinol Diabetes 104 (Suppl. 3):17 –20,1996 [DOI] [PubMed] [Google Scholar]
- 30.Salomon B, Rhee L, Bour-Jordan H, Hsin H, Montag A, Soliven B, Arcella J, Girvin AM, Padilla J, Miller SD, Bluestone JA: Development of spontaneous autoimmune peripheral polyneuropathy in B7–2-deficient NOD mice. J Exp Med 194 :677 –684,2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Beales PE, Castri F, Valiant A, Rosignoli G, Buckley L, Pozzilli P: Adrenalitis in the non-obese diabetic mouse. Autoimmunity 35 :329 –333,2002 [DOI] [PubMed] [Google Scholar]
- 32.Carrasco-Marin E, Shimizu J, Kanagawa O, Unanue ER: The class II MHC I-Ag7 molecules from non-obese diabetic mice are poor peptide binders. J Immunol 156 :450 –458,1996 [PubMed] [Google Scholar]
- 33.Kishimoto H, Sprent J: A defect in central tolerance in NOD mice. Nat Immunol 2 :1025 –1031,2001 [DOI] [PubMed] [Google Scholar]
- 34.Lesage S, Hartley SB, Akkaraju S, Wilson J, Townsend M, Goodnow CC: Failure to censor forbidden clones of CD4 T cells in autoimmune diabetes. J Exp Med 196 :1175 –1188,2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Zucchelli S, Holler P, Yamagata T, Roy M, Benoist C, Mathis D: Defective central tolerance induction in NOD mice: genomics and genetics. Immunity 22 :385 –396,2005 [DOI] [PubMed] [Google Scholar]
- 36.Jolicoeur C, Hanahan D, Smith KM: T-cell tolerance toward a transgenic beta-cell antigen and transcription of endogenous pancreatic genes in thymus. Proc Natl Acad Sci U S A 91 :6707 –6711,1994 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Chentoufi AA, Polychronakos C: Insulin expression levels in the thymus modulate insulin-specific autoreactive T-cell tolerance: the mechanism by which the IDDM2 locus may predispose to diabetes. Diabetes 51 :1383 –1390,2002 [DOI] [PubMed] [Google Scholar]
- 38.Anderson MS, Venanzi ES, Klein L, Chen Z, Berzins SP, Turley SJ, von Boehmer H, Bronson R, Dierich A, Benoist C, Mathis D: Projection of an immunological self shadow within the thymus by the aire protein. Science 298 :1395 –1401,2002 [DOI] [PubMed] [Google Scholar]
- 39.Semana G, Gausling R, Jackson RA, Hafler DA: T cell autoreactivity to proinsulin epitopes in diabetic patients and healthy subjects. J Autoimmun 12 :259 –267,1999 [DOI] [PubMed] [Google Scholar]
- 40.Durinovic-Bello I, Boehm BO, Ziegler AG: Predominantly recognized proinsulin T helper cell epitopes in individuals with and without islet cell autoimmunity. J Autoimmun 18 :55 –66,2002 [DOI] [PubMed] [Google Scholar]
- 41.Arif S, Tree TI, Astill TP, Tremble JM, Bishop AJ, Dayan CM, Roep BO, Peakman M: Autoreactive T cell responses show proinflammatory polarization in diabetes but a regulatory phenotype in health. J Clin Invest 113 :451 –463,2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Toma A, Haddouk S, Briand JP, Camoin L, Gahery H, Connan F, Dubois-Laforgue D, Caillat-Zucman S, Guillet JG, Carel JC, Muller S, Choppin J, Boitard C: Recognition of a subregion of human proinsulin by class I-restricted T cells in type 1 diabetic patients. Proc Natl Acad Sci U S A 102 :10581 –10586,2005 [DOI] [PMC free article] [PubMed] [Google Scholar]