Abstract
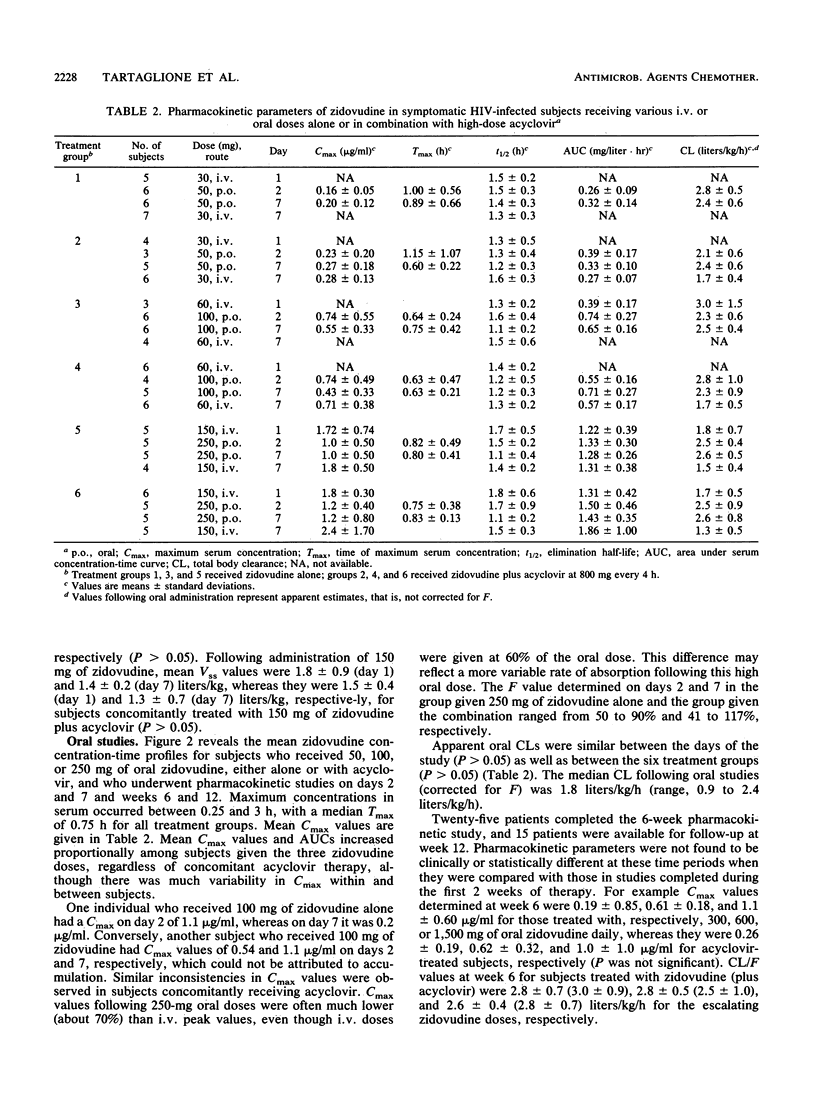
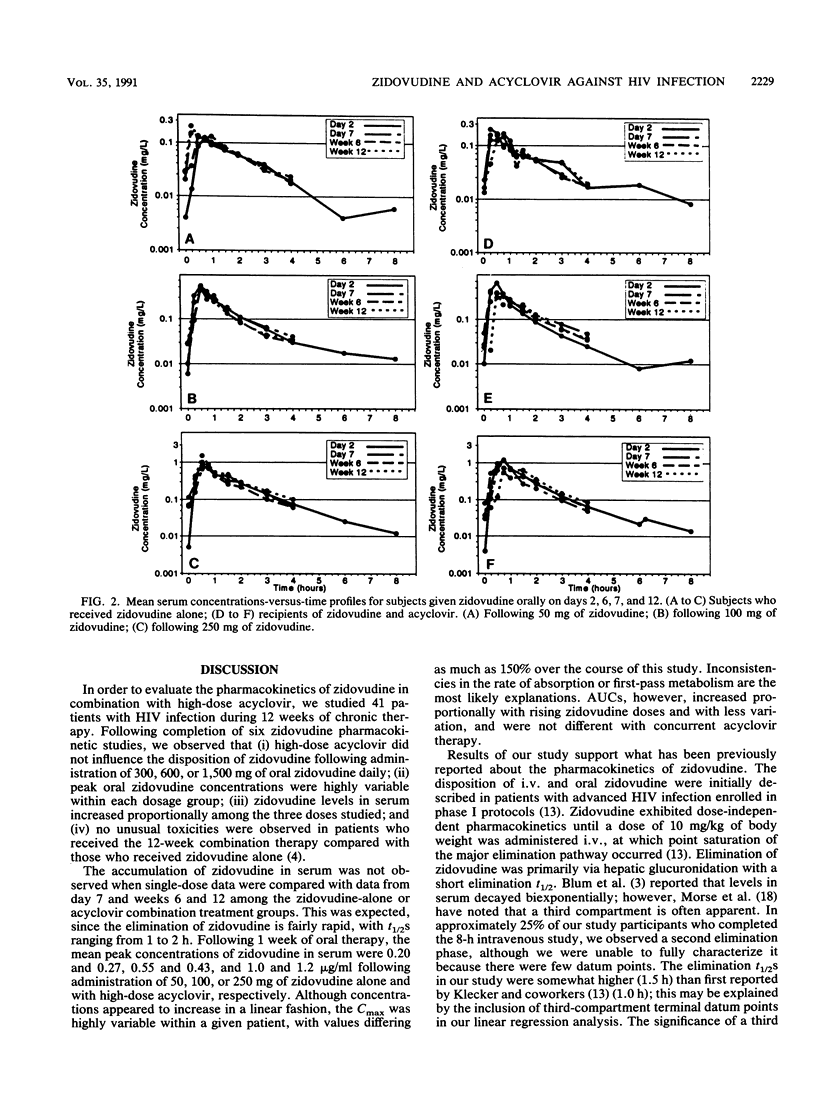
The pharmacokinetics of zidovudine were evaluated in 41 patients with Centers for Disease Control HIV class IVA infection. The patients were assigned escalating doses of zidovudine (300, 600, or 1,500 mg daily) and were randomized to receive either zidovudine alone or zidovudine with a high dose of acyclovir (4,800 mg per day). Single and multiple intravenous- and oral-dose pharmacokinetic studies were performed on days 1 and 7 and weeks 6 and 12 of therapy. Zidovudine concentrations were analyzed by high-pressure liquid chromatography. Pharmacokinetic parameters were estimated by noncompartmental methods. Zidovudine concentrations in serum declined in a biphasic manner, with half-lives ranging from 1 to 2 h, and were independent of acyclovir administration or length of zidovudine therapy. The median time of peak concentrations in serum following oral doses was 0.75 h (range, 0.25 to 3 h). Accumulation of zidovudine in serum was not observed, but the maximum concentration of drug in serum (Cmax) and the area under the concentration-time curve increased proportionally with increased zidovudine doses. Mean day 7 oral Cmax values were 0.20 +/- 0.12, 0.55 +/- 0.33, and 1.0 +/- 0.5 micrograms/ml for 17 patients receiving total daily doses of, respectively, 300, 600, and 1,500 mg of zidovudine alone, whereas Cmax values were, respectively, 0.27 +/- 0.18, 0.43 +/- 0.33, and 1.2 +/- 0.80 micrograms/ml for 15 comparably treated recipients of zidovudine plus acyclovir (P was not significant). The median bioavailability of oral zidovudine was 67% (42 to 120%) and did not vary with dosage. Absolute and apparent total body clearances were similar among the patients given the various zidovudine doses regardless of whether there was concomitant acyclovir therapy. Drug-related toxicities were observed more frequently in the subjects who received high doses of zidovudine than they were in those who received median and low doses of zidovudine (P=0.03). Overall, acyclovir did not influence the disposition of zidovudine over a wide range of zidovudine doses. No unusual toxicities could be attributed to the zidovudine and high-dose acyclovir combination during the 12-week observation period.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bach M. C. Possible drug interaction during therapy with azidothymidine and acyclovir for AIDS. N Engl J Med. 1987 Feb 26;316(9):547–547. doi: 10.1056/NEJM198702263160912. [DOI] [PubMed] [Google Scholar]
- Bauer L. A., Gibaldi M. Computation of model-independent pharmacokinetic parameters during multiple dosing. J Pharm Sci. 1983 Aug;72(8):978–979. doi: 10.1002/jps.2600720843. [DOI] [PubMed] [Google Scholar]
- Blum M. R., Liao S. H., Good S. S., de Miranda P. Pharmacokinetics and bioavailability of zidovudine in humans. Am J Med. 1988 Aug 29;85(2A):189–194. [PubMed] [Google Scholar]
- Collier A. C., Bozzette S., Coombs R. W., Causey D. M., Schoenfeld D. A., Spector S. A., Pettinelli C. B., Davies G., Richman D. D., Leedom J. M. A pilot study of low-dose zidovudine in human immunodeficiency virus infection. N Engl J Med. 1990 Oct 11;323(15):1015–1021. doi: 10.1056/NEJM199010113231502. [DOI] [PubMed] [Google Scholar]
- Creagh-Kirk T., Doi P., Andrews E., Nusinoff-Lehrman S., Tilson H., Hoth D., Barry D. W. Survival experience among patients with AIDS receiving zidovudine. Follow-up of patients in a compassionate plea program. JAMA. 1988 Nov 25;260(20):3009–3015. [PubMed] [Google Scholar]
- Dunne A. JANA: a new iterative polyexponential curve stripping program. Comput Methods Programs Biomed. 1985 Aug;20(3):269–275. doi: 10.1016/0169-2607(85)90085-9. [DOI] [PubMed] [Google Scholar]
- Fiddian A. P. Preliminary report of a multicentre study of zidovudine plus or minus acyclovir in patients with acquired immune deficiency syndrome or acquired immune deficiency syndrome-related complex. J Infect. 1989 Jan;18 (Suppl 1):79–80. doi: 10.1016/s0163-4453(89)80084-2. [DOI] [PubMed] [Google Scholar]
- Fischl M. A., Richman D. D., Grieco M. H., Gottlieb M. S., Volberding P. A., Laskin O. L., Leedom J. M., Groopman J. E., Mildvan D., Schooley R. T. The efficacy of azidothymidine (AZT) in the treatment of patients with AIDS and AIDS-related complex. A double-blind, placebo-controlled trial. N Engl J Med. 1987 Jul 23;317(4):185–191. doi: 10.1056/NEJM198707233170401. [DOI] [PubMed] [Google Scholar]
- Gitterman S. R., Drusano G. L., Egorin M. J., Standiford H. C. Population pharmacokinetics of zidovudine. The Veterans Administration Cooperative Studies Group. Clin Pharmacol Ther. 1990 Aug;48(2):161–167. doi: 10.1038/clpt.1990.131. [DOI] [PubMed] [Google Scholar]
- Good S. S., Reynolds D. J., de Miranda P. Simultaneous quantification of zidovudine and its glucuronide in serum by high-performance liquid chromatography. J Chromatogr. 1988 Sep 23;431(1):123–133. doi: 10.1016/s0378-4347(00)83075-3. [DOI] [PubMed] [Google Scholar]
- Klecker R. W., Jr, Collins J. M., Yarchoan R., Thomas R., Jenkins J. F., Broder S., Myers C. E. Plasma and cerebrospinal fluid pharmacokinetics of 3'-azido-3'-deoxythymidine: a novel pyrimidine analog with potential application for the treatment of patients with AIDS and related diseases. Clin Pharmacol Ther. 1987 Apr;41(4):407–412. doi: 10.1038/clpt.1987.49. [DOI] [PubMed] [Google Scholar]
- Laskin O. L., Longstreth J. A., Saral R., de Miranda P., Keeney R., Lietman P. S. Pharmacokinetics and tolerance of acyclovir, a new anti-herpesvirus agent, in humans. Antimicrob Agents Chemother. 1982 Mar;21(3):393–398. doi: 10.1128/aac.21.3.393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitsuya H., Weinhold K. J., Furman P. A., St Clair M. H., Lehrman S. N., Gallo R. C., Bolognesi D., Barry D. W., Broder S. 3'-Azido-3'-deoxythymidine (BW A509U): an antiviral agent that inhibits the infectivity and cytopathic effect of human T-lymphotropic virus type III/lymphadenopathy-associated virus in vitro. Proc Natl Acad Sci U S A. 1985 Oct;82(20):7096–7100. doi: 10.1073/pnas.82.20.7096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morse G. D., Portmore A., Olson J., Taylor C., Plank C., Reichman R. C. Multiple-dose pharmacokinetics of oral zidovudine in hemophilia patients with human immunodeficiency virus infection. Antimicrob Agents Chemother. 1990 Mar;34(3):394–397. doi: 10.1128/aac.34.3.394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spector S. A., Connor J. D., Hintz M., Quinn R. P., Blum M. R., Keeney R. E. Single-dose pharmacokinetics of acyclovir. Antimicrob Agents Chemother. 1981 Apr;19(4):608–612. doi: 10.1128/aac.19.4.608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Volberding P. A., Lagakos S. W., Koch M. A., Pettinelli C., Myers M. W., Booth D. K., Balfour H. H., Jr, Reichman R. C., Bartlett J. A., Hirsch M. S. Zidovudine in asymptomatic human immunodeficiency virus infection. A controlled trial in persons with fewer than 500 CD4-positive cells per cubic millimeter. The AIDS Clinical Trials Group of the National Institute of Allergy and Infectious Diseases. N Engl J Med. 1990 Apr 5;322(14):941–949. doi: 10.1056/NEJM199004053221401. [DOI] [PubMed] [Google Scholar]
- Wade J. C., Meyers J. D. Neurologic symptoms associated with parenteral acyclovir treatment after marrow transplantation. Ann Intern Med. 1983 Jun;98(6):921–925. doi: 10.7326/0003-4819-98-6-921. [DOI] [PubMed] [Google Scholar]
- Whitley R. J., Blum M. R., Barton N., de Miranda P. Pharmacokinetics of acyclovir in humans following intravenous administration. A model for the development of parenteral antivirals. Am J Med. 1982 Jul 20;73(1A):165–171. doi: 10.1016/0002-9343(82)90084-5. [DOI] [PubMed] [Google Scholar]
- de Miranda P., Good S. S., Laskin O. L., Krasny H. C., Connor J. D., Lietman P. S. Disposition of intravenous radioactive acyclovir. Clin Pharmacol Ther. 1981 Nov;30(5):662–672. doi: 10.1038/clpt.1981.218. [DOI] [PubMed] [Google Scholar]


