Abstract
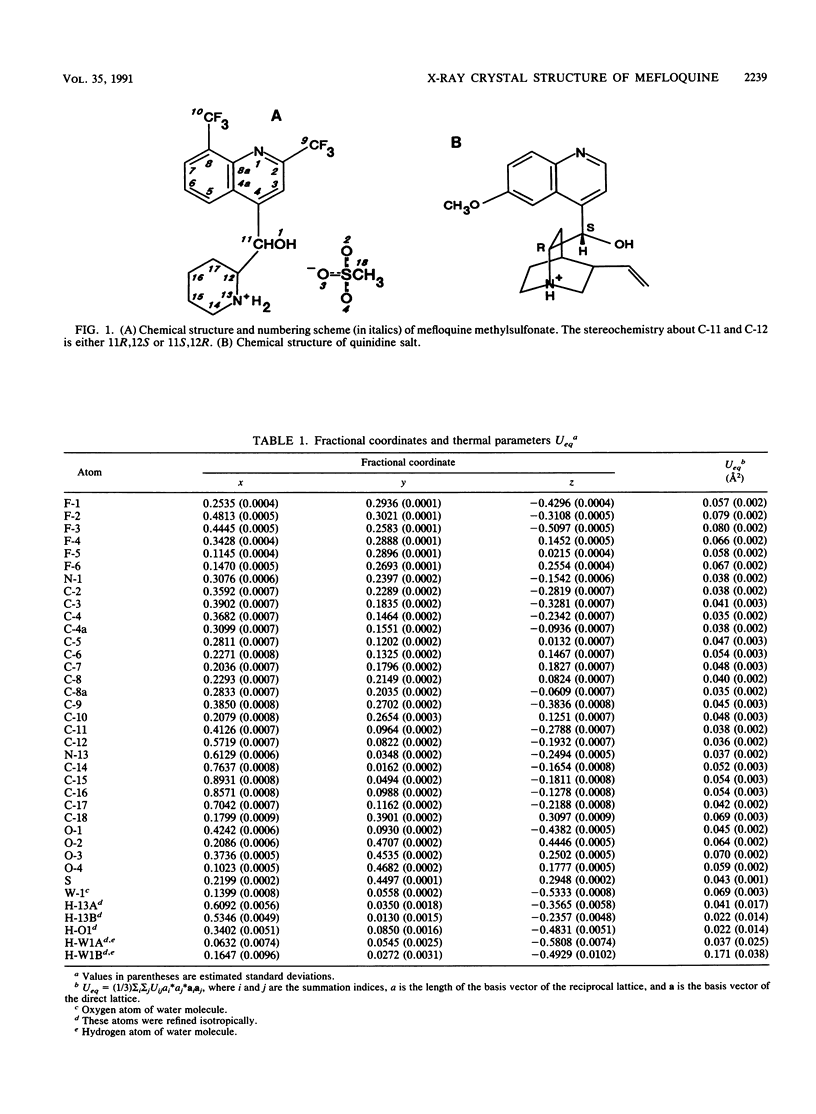
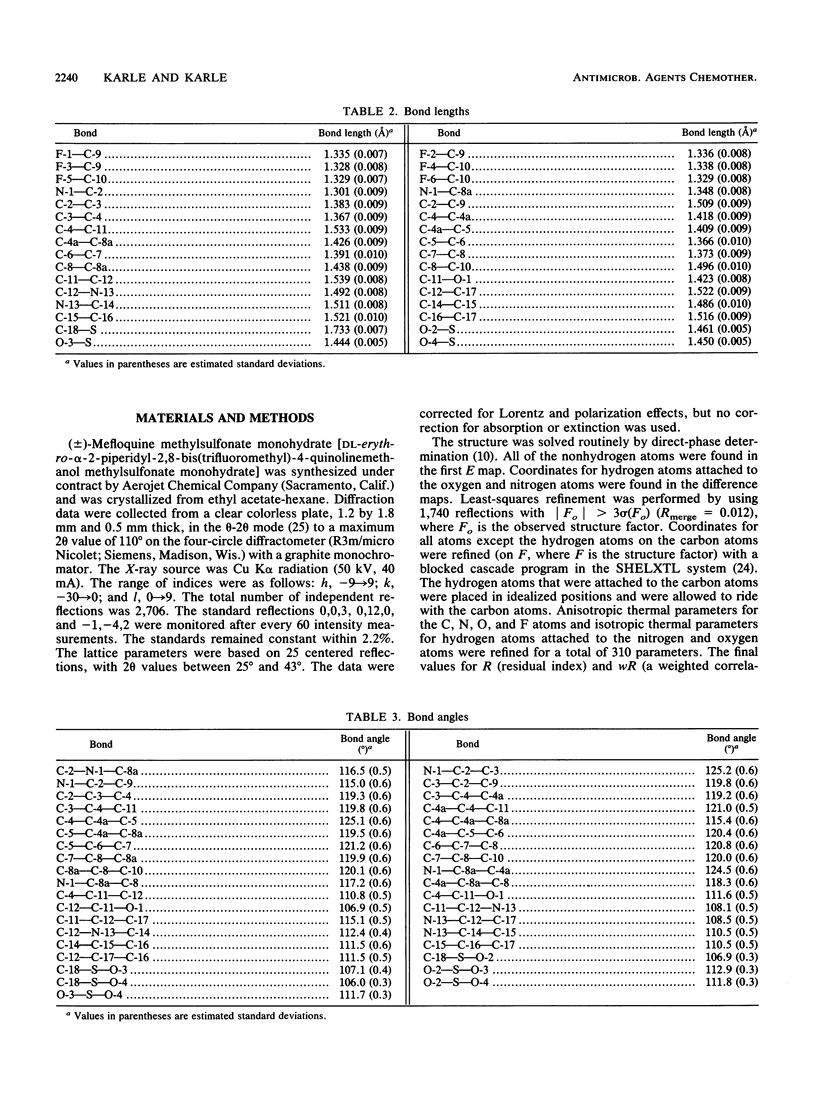
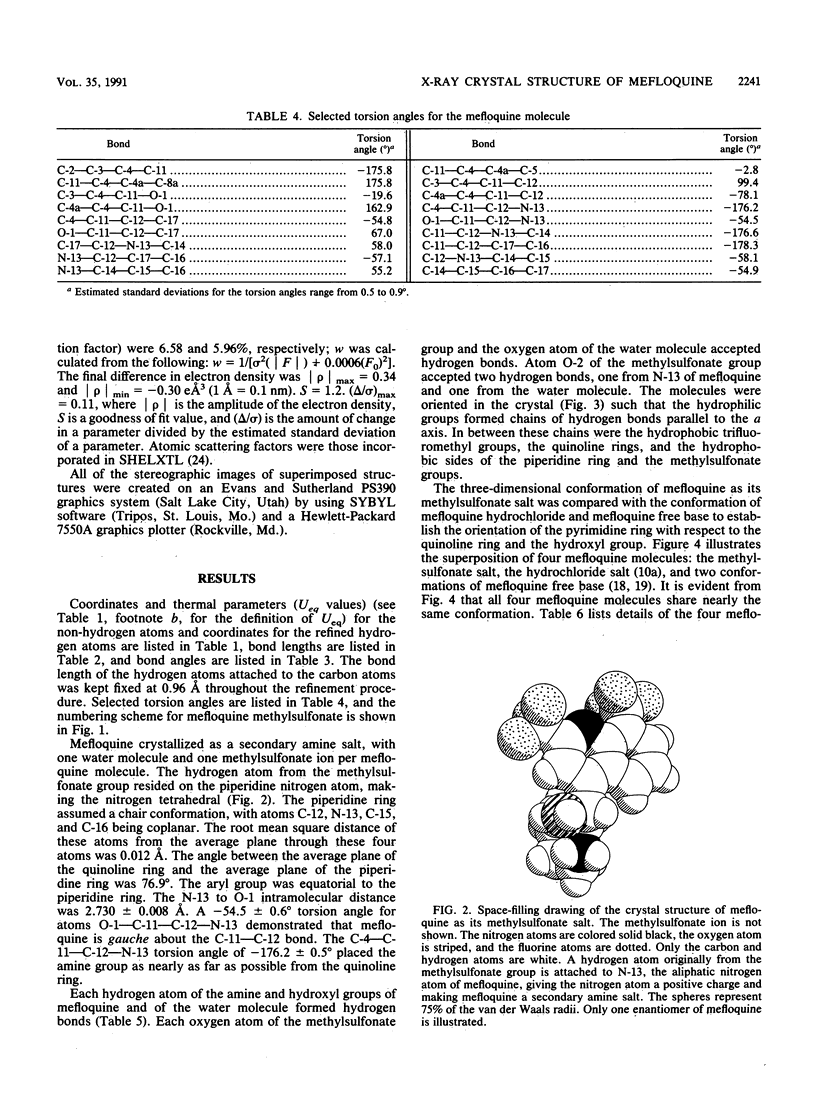
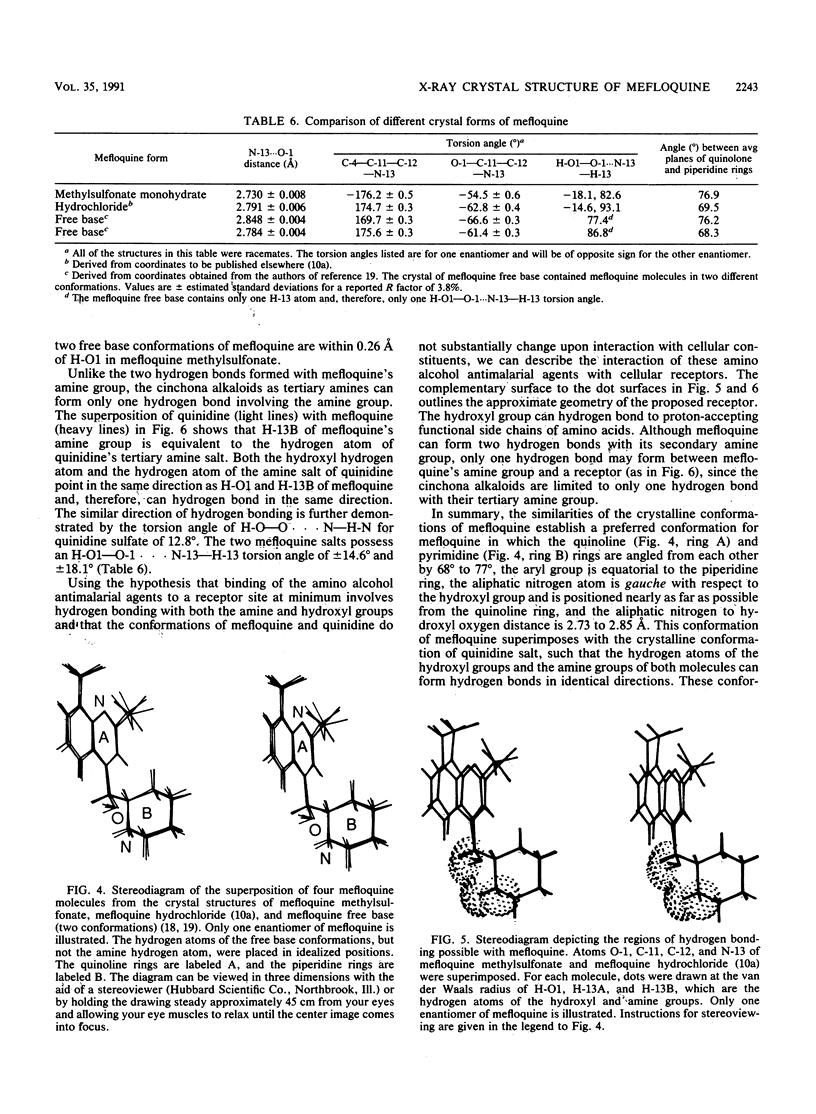
The crystal structure of (+/-)-mefloquine methylsulfonate monohydrate was determined by X-ray diffraction and was compared with the crystal structures of mefloquine hydrochloride and mefloquine free base. The conformation of mefloquine was essentially the same in all three crystalline environments and was not dependent on whether mefloquine was a salt or a free base. In mefloquine methylsulfonate monohydrate, the angle between the average plane of the quinoline ring and the average plane of the piperidine ring was 76.9 degrees. The intramolecular aliphatic N-13...O-1 distance was 2.730 +/- 0.008 A (1 A = 0.1 nm), which is close to the aliphatic N...O distance found in the antimalarial cinchona alkaloids. The hydroxyl group formed a hydrogen bond with the water molecule, and the amine group formed hydrogen bonds with two different methylsulfonate ions. The crystallographic parameters for (+/-)-mefloquine methylsulfonate monohydrate were as follows: C17H17F6N2O(+).CH3SO3(-).H2O; Mr = 492.4; symmetry of unit cell, monoclinic; space group, P2(1)/a; parameters of unit cell, a was 8.678 +/- 0.001 A, b was 28.330 +/- 0.003 A, c was 8.804 +/- 0.001 A, beta was 97.50 +/- 0.01 degrees; the volume of the unit cell was 2145.9 A3; the number of molecules per unit cell was 4; the calculated density was 1.52 g cm(-3); the source of radiation was Cu K alpha (lambda = 1.54178 A); mu (absorption coefficient) was 20.46 cm(-1); F(000) (sum of atomic scattering factors at zero scattering angle) was 1,016; room temperature was used; and the final R (residual index) was 6.58% for 1,740 reflections with magnitude of Fo greater than 3 sigma (F). Since the mechanism of antimalarial action and the mechanism of mefloquine resistance may involve hydrogen bond formation between mefloquine and a cellular effector or transport proteins, the common conformation of mefloquine found in each crystalline environment may define the orientation in which mefloquine forms these potentially critical hydrogen bonds with cellular constituents.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Boudreau E. F., Webster H. K., Pavanand K., Thosingha L. Type II mefloquine resistance in Thailand. Lancet. 1982 Dec 11;2(8311):1335–1335. doi: 10.1016/s0140-6736(82)91532-x. [DOI] [PubMed] [Google Scholar]
- Carroll F. I., Blackwell J. T. Optical isomers of aryl-2-piperidylmethanol antimalarial agents. Preparation, optical purity, and absolute stereochemistry. J Med Chem. 1974 Feb;17(2):210–219. doi: 10.1021/jm00248a015. [DOI] [PubMed] [Google Scholar]
- Chien P. L., Cheng C. C. Difference in antimalarial activity between certain amino alcohol diastereomers. J Med Chem. 1976 Jan;19(1):170–172. doi: 10.1021/jm00223a032. [DOI] [PubMed] [Google Scholar]
- Chien P. L., Cheng C. C. Further side-chain modification of antimalarial phenanthrene amino alcohols. J Med Chem. 1973 Oct;16(10):1093–1096. doi: 10.1021/jm00268a006. [DOI] [PubMed] [Google Scholar]
- Doherty R., Benson W. R., Maienthal M., Stewart J. M. Crystal and molecular structure of quinidine. J Pharm Sci. 1978 Dec;67(12):1698–1701. doi: 10.1002/jps.2600671217. [DOI] [PubMed] [Google Scholar]
- Félix R., Gay F., Lyogoubi A., Bustos M. D., Diquet B., Danis M., Gentilini M. Résistance croisée à la méfloquine et à l'halofantrine lors d'un paludisme à P. falciparum contracté en Sierra Leone. Bull Soc Pathol Exot. 1990;83(1):43–45. [PubMed] [Google Scholar]
- Karle I. L., Karle J. Anomalous dispersion of sulfur in quinidine sulfate, (C(20)H(25)N(2)O(2))(2)SO(4).2H(2)O: Implications for structure analysis. Proc Natl Acad Sci U S A. 1981 Oct;78(10):5938–5941. doi: 10.1073/pnas.78.10.5938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karwacki J. J., Webster H. K., Limsomwong N., Shanks G. D. Two cases of mefloquine resistant malaria in Thailand. Trans R Soc Trop Med Hyg. 1989 Mar-Apr;83(2):152–153. doi: 10.1016/0035-9203(89)90620-2. [DOI] [PubMed] [Google Scholar]
- Krogstad D. J., Schlesinger P. H., Gluzman I. Y. Antimalarials increase vesicle pH in Plasmodium falciparum. J Cell Biol. 1985 Dec;101(6):2302–2309. doi: 10.1083/jcb.101.6.2302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olsen R. E. Antimalarial activity and conformation of erythro- and threo- -(2-piperidyl)-3,6-bis(trifluoromethyl)-9-phenanthrenemethanol. J Med Chem. 1972 Feb;15(2):207–208. doi: 10.1021/jm00272a024. [DOI] [PubMed] [Google Scholar]
- Panisko D. M., Keystone J. S. Treatment of malaria--1990. Drugs. 1990 Feb;39(2):160–189. doi: 10.2165/00003495-199039020-00002. [DOI] [PubMed] [Google Scholar]
- Payne D. Spread of chloroquine resistance in Plasmodium falciparum. Parasitol Today. 1987 Aug;3(8):241–246. doi: 10.1016/0169-4758(87)90147-5. [DOI] [PubMed] [Google Scholar]
- Sweeney T. R. The present status of malaria chemotherapy: mefloquine, a novel antimalarial. Med Res Rev. 1981 Fall;1(3):281–301. doi: 10.1002/med.2610010304. [DOI] [PubMed] [Google Scholar]


