Abstract
The neonatal Fc receptor for IgG (FcRn) is a distant member of the MHC class I protein family. It binds IgG and albumin in a pH-dependent manner and protects these from catabolism by diverting them from a degradative fate in lysosomes. In addition, FcRn-mediated IgG transport across epithelial barriers is responsible for the transmission of IgG from mother to infant and can also enhance IgG-mediated antigen uptake across mucosal epithelia. We now show a previously undescribed role for FcRn in mediating the presentation of antigens by dendritic cells when antigens are present as a complex with antibody by uniquely directing multimeric immune complexes, but not monomeric IgG, to lysosomes.
Keywords: IgG, immune complexes, dendritic cells
Professional antigen (Ag)-presenting cells (APC) such as macrophages and dendritic cells (DC) are specialized cells that take up extracellular Ag either by nonspecific pinocytosis or more efficiently by receptor-mediated uptake. After processing in acidified endosomes and lysosomes, Ag fragments are loaded onto MHC class II molecules and presented to T cells. DCs express several types of Fcγ receptors (FcγR) that bind the Fc portion of IgG molecules with different binding affinities (1–4). Ags incorporated in immune complexes (ICs) are taken up and presented by DC more efficiently than soluble Ags alone, an effect that has been attributed to the stimulatory signals mediated by activating FcγRs and increased uptake efficiency of Ag when receptor-mediated uptake via FcγR, in particular FcγRII, is engaged (2).
The neonatal Fc receptor for IgG (FcRn) is an Fc-binding molecule that is structurally and functionally different from the FcγR. FcRn is related to the MHC class I protein and consists of a glycosylated heavy chain in noncovalent association with β2-microglobulin (5). FcRn binds its two major ligands, IgG and serum albumin, in a pH-dependent manner, in which efficient binding is seen only at acidic pH <6.5 and not at neutral pH >7.0 (6, 7). The FcRn-binding site on IgG involves several histidine residues at the CH2-CH3 domain interface of the Fc fragment and is distinct from binding sites for FcγR (8, 9). The main biological functions for FcRn have been identified to be protecting IgG from catabolism by diverting bound IgG molecules away from a degradative fate in lysosomes, a similar protective function for serum albumin, and transport of IgG across epithelial and endothelial barriers that is responsible for transmission of IgG from mother to infant and IgG mediated uptake of Ags across epithelial barriers (7, 10–12).
In rodents, intestinal expression of FcRn within the epithelium is down-regulated upon weaning, whereas human intestinal epithelial cells continue to express FcRn into adulthood (13). In addition, human FcRn is expressed in other adult parenchymal cells such as kidney and bronchial epithelial and endothelial cells (14, 15). The expression of human FcRn has been identified in hematopoietic cells such as small intestinal macrophages, monocytes, and monocyte-derived DC but not other closely related immune cells such as B and T cells (16). Similar to human APC (16), adult murine DC express FcRn (17). The specific expression of this recently discovered Fc-binding receptor in professional APC prompted us to investigate whether FcRn may be involved in the uptake and presentation of Fc-containing Ags. In this study, we show that FcRn in human and murine DC enhances the uptake and presentation of Ag-antibody ICs indicative of a previously uncharacterized function for FcRn; a role in adaptive immunity.
Results
FcRn Is Expressed in Adult Murine APC.
Similar to earlier reports of human FcRn expression in professional APC (16), we observed FcRn mRNA expression in adult WT B6 murine bone marrow (BM), macrophages, and BM-derived DC [supporting information (SI) Fig. S1A]. Western blot analysis of WT splenic DC lysates confirmed FcRn protein expression as defined by a 50-kDa band that was absent in DC lysates from FcRn-deficient mice (Fig. S1B). These studies show that FcRn is expressed in both mouse and human DC in agreement with previous reports (16, 17).
FcRn Enhances Ag Presentation of Murine DC Both in Vitro and in Vivo.
We next sought to determine whether FcRn within mouse DC affected Ag presentation. We took advantage of the fact that mouse FcRn is able to bind human IgG1 Fc with high affinity (18). We used a chimeric antibody that contains a murine Fab specific for the hapten 4-hydroxy-3-iodo-5-nitrophenylacetic acid (NIP) and WT Fc derived from human IgG1 (19). We further engineered this chimeric antibody to bear Fc mutations in three critical amino acids [I253A, H310A, and H435A (IHH)] (14) that disable binding to murine (20, 21) and human FcRn (22). As shown in Fig. 1, whereas the WT form of this chimeric antibody could interact with FcRn from human DC at pH 6, but not at pH 8, the IHH mutant isoform could not bind FcRn under the same conditions at pH 6. A 25-kDa band corresponding to the light chain of the NIPIgG antibody used to pull down the FcRn was present in equal amounts under all conditions (Fig. 1A), showing that IHH mutation does not affect protein G binding. The IHH NIPIgG mutant also exhibited the same binding affinity to FcγR as the WT isoform when tested in an ELISA using soluble human FcγRII and FcγRI molecules; the two isoforms expressed by human DC (Fig. 1 B and D) (1). This is in accordance with previous studies showing that the I253A and/or H435A mutations inhibit FcRn binding but have no effect on FcγR binding (9, 22).
Fig. 1.
The IHH mutation abrogates FcRn binding but does not affect binding to FcγR. (A) Human monocyte-derived DC lysates were subjected to sequential binding with WT or IHH-mutated NIPIgG and protein G beads at pH 6 or 8. FcRn protein was detected as a 40-kDa band in a human FcRn-specific immunoblot. The light chain of NIPIgG was also detected (arrowhead). (B) WT (square) or IHH-mutated (triangle) NIPIgG were tested in an ELISA for binding affinity to recombinant GST-tagged human FcRn at pH 5.5, (C) to human FcγRI, and (D) to human FcγRIIa at pH 7.4.
In an in vitro T cell proliferation assay, we observed that the OVA323–339 T cell epitope was more efficiently presented by WT murine DC to OT-II T cells compared with that observed with FcRn-deficient DC when NIP-conjugated ovalbumin (NIP-OVA, on average 15 NIP molecules per OVA protein) was provided as an IC with the engineered chimeric NIPIgG (Fig. 2A). When NIP-OVA was presented as a soluble Ag, there was no difference between WT and FcRn-deficient DC in the presentation of the OVA323–339 epitope (Fig. 2A). Similarly, the presentation of this epitope was reduced when it was contained within ICs that were incapable of binding FcRn (IHH-mutated NIPIgG) relative to WT ICs and was unaffected by the presence or absence of FcRn expression by the DC (Fig. 2B). Thus, when FcRn function is absent either by deletion of FcRn in the DC or abrogation of the ability of IgG to bind to FcRn, the presentation of Ag as an IC is significantly decreased.
Fig. 2.
Enhanced in vitro presentation of FcRn-binding OVA-ICs by FcRn-expressing DC. Spleen DC from WT mice (filled symbols) or FcRn-deficient (KO) mice (open symbols) were used to present NIP-OVA (triangles), ICs of WT NIPIgG and NIP-OVA (squares), or non-FcRn-binding ICs of IHH-mutated NIPIgG and NIP-OVA (circles) to an OVA-reactive OT-II T cell line. Error bars indicate the observed range within triplicates in one representative experiment of four. WT DC presenting WT ICs was statistically significantly different from all other APC and Ag combinations (P < 0.01) and FcRn-deficient DC presenting WT ICs was significantly different from FcRn-deficient DC-presenting soluble NIP-OVA (P < 0.05), whereas all of the other Ag conditions did not display statistically significant differences from each other in one-way ANOVA with Bonferroni post test (P > 0.05).
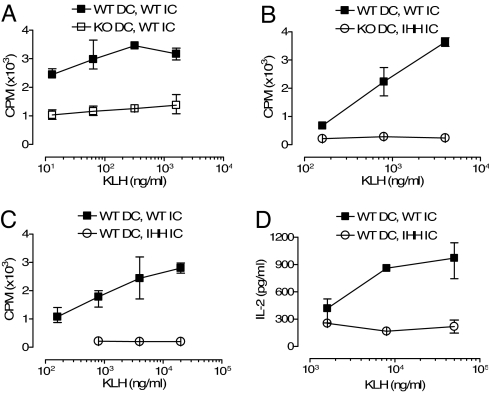
To determine whether FcRn regulates Ag presentation by DC in vivo, we injected FcRn-expressing or -deficient DC loaded in vitro with FcRn-binding or nonbinding ICs containing the keyhole limpet hemocyanin (KLH) Ag into the hind footpads of WT B6 mice. Five days later, the extent of in vivo Ag presentation was examined by assessing IL-2 secretion and proliferation of T cells obtained from the draining popliteal lymph nodes as defined by a recall response to KLH. In this assay, the precursor frequency of KLH responsive naïve T cells is very low, and the Ag stimulation threshold needed to elicit T cell responses is relatively high. Consistent with this, when DC were incubated overnight with 50 μg/ml NIP-KLH alone, washed, and injected s.c. into the footpads of WT mice, these Ag-loaded DC were unable to initiate a T cell response in the recipient naïve mice as assessed by in vitro recall assays (data not shown). In comparison, when WT DC were incubated overnight with FcRn-binding KLH ICs, washed, and then injected into the footpads, the Ag-loaded DC were able to initiate a robust T cell response to KLH as assessed by a concentration-dependent increase in 3H-thymidine incorporation (filled symbols, Fig. 3 A and C) and IL-2 secretion (filled symbols, Fig. 3D). In contrast, when FcRn-deficient DC loaded with the same KLH-ICs (open symbols, Fig. 3A) or WT DC loaded with IHH-mutated KLH-ICs (Fig. 3 C and D) were injected into the contralateral footpads of the same animal, the Ag presentation was markedly blunted, as evidenced by the largely absent in vitro recall responses to KLH. These studies show that FcRn function is critical for allowing a DC to present ICs to naïve T cells and initiate a T cell response in vivo.
Fig. 3.
Enhanced in vivo presentation of FcRn-binding KLH-ICs by in vitro Ag-loaded DC. Spleen DC from WT (filled symbols) or FcRn-deficient (KO, open symbols, A and B) mice loaded in vitro overnight with Ags were injected into hind footpads of WT B6 mice. Ags used were ICs of WT NIPIgG + NIP-KLH (filled symbols) or IHH-mutated NIPIgG + NIP-KLH (open symbols, B–D). Five days after injection, draining popliteal lymph nodes were assayed in vitro for T cell recall response to KLH. Error bars indicate the observed range within triplicates in one representative experiment of three. All pairs of closed and open graphs were significantly different in A–D (Mann–Whitney test, P < 0.05).
To confirm these observations, we used an in vivo T cell proliferation assay to directly assess the effect of FcRn on Ag presentation. Immediately after i.v. transfer of carboxyfluorescein succinimidyl ester (CFSE)-labeled naïve OVA-specific DO11.10 T cells, the recipient WT or FcRn-deficient Balb/C mice were immunized s.c. with WT OVA-ICs in the left hind footpad (L) and with IHH-mutated and thus FcRn nonbinding OVA-ICs in the right hind footpad (R) of the same animal. The in vivo proliferation of the labeled DO11.10 T cells as a consequence of the uptake, processing, and presentation of the OVA-containing ICs by the host APC were then tracked by CFSE dilution of the transferred T cells in the draining popliteal lymph nodes. Because both sets of the functional and nonfunctional ICs contained similar amounts of OVA, it was observed that the highly OVA-reactive DO11.10 T cells proliferated vigorously in both sets of popliteal lymph nodes (Fig. S2). Therefore, to compare the effect of FcRn on Ag presentation, we chose to evaluate the extent of T cell proliferation in each lymph node sample with the calculated theoretical number of mitosis undergone by each progenitor cell, called mitosis/progenitor ratio (M/P ratio) (Fig. S3) from a large group of mice (23). In WT mice, the labeled DO11.10 T cells recruited to the left-side popliteal lymph nodes (L) draining FcRn binding, WT OVA ICs were observed to undergo more cell divisions compared with the same cells that were recruited to the right side (R) immunized with non-FcRn binding, IHH-mutated OVA-ICs (Fig. 4). In contrast, in FcRn-deficient animals, no significant differences were observed in DO11.10 T cell divisions elicited by either type of FcRn-binding or nonbinding OVA ICs (Fig. 4). These results show that the ability of an IC to be presented by a professional APC and to activate a T cell is significantly enhanced by its ability to bind FcRn.
Fig. 4.
Enhanced in vivo Ag presentation and T cell proliferation of FcRn-binding ICs in WT mice. The proliferation of CFSE-labeled DO11.10 CD4 T cells was assessed in draining popliteal lymph nodes of WT and FcRn-deficient (KO) Balb/C mice 3–5 days after transfer and Ag immunization in hind footpads. Ags used were ICs of WT NIPIgG + NIP-OVA on the left side (L) or IHH-mutated NIPIgG + NIP-OVA on the right side (R). The mitosis per progenitor cell (M/P) ratio was calculated, and a Wilcoxon paired test was performed for each group of mice.
FcRn in Human APC Enhances the Presentation of Large IgG–Ag Complexes.
We next sought to determine whether FcRn regulated the ability of human APC to present Ag. We first tested DC presentation of monomeric IgG–Ag complexes prepared with NIPIgG and peptides conjugated with one NIP molecule N-terminally. When presented by HLA-DQ2 molecules, a gliadin-derived T cell epitope contained within this peptide can be recognized by intestinal T cells from celiac disease patients (24). DC derived from HLA-DQ2-positive monocytes did not present the monomeric WT IgG–peptide complexes any more effectively than the monomeric IHH-mutated IgG–peptide complexes or the peptide alone (Fig. 5A). To prepare multimeric ICs similar to the OVA ICs used in the murine assays, we covalently conjugated gliadin protein with NIP molecules by treating gliadin with Osu-NIP. The T cell epitope within the multimeric WT NIP-gliadin ICs was shown to be more effectively presented to T cells compared with multimeric IHH-mutated ICs that were unable to bind FcRn (Fig. 5B). To further demonstrate that FcRn-binding multimeric ICs were more efficiently presented, we synthesized a peptide conjugated with two NIP molecules such that it would form multimeric ICs upon incubation with NIPIgG. The presentation of FcRn-binding ICs formed by WT NIPIgG and these dual NIP-conjugated peptides by DC was indeed enhanced compared with that of similar ICs containing IHH-mutated IgG and thus were unable to bind FcRn. Importantly, the presentation of both classes of ICs by EBV transformed B cells that do not express FcRn were equal (Fig. 4C). These studies show that FcRn also regulates Ag presentation by human DC, as shown with mouse DC when the Ag and antibody are present as multimeric ICs.
Fig. 5.
Selective FcRn-mediated presentation enhancement of multimeric ICs in human in vitro T cell proliferation assay. Monocyte-derived DC from an HLA-DQ2 donor were used to present monomeric IgG-Ag molecules (A) or multimeric ICs (B) to a CD4 T cell clone reactive to the IQPEPQPAQL peptide presented on HLA-DQ2 molecules. NIP-peptide (NIP-CLRMKLGIIQPEQPAQL) or NIP-gliadin was used alone (filled triangle) or in complex with 200 μg/ml WT NIPIgG (filled square) or IHH-mutated NIPIgG (filled circle). Error bars indicate the observed range within triplicates in one representative experiment of three. NIP-peptide alone was statistically significantly different from both NIPIgG + NIP-peptide conditions (A) (P < 0.05) and WT NIPIgG + NIP-gliadin was statistically significant from the other two Ags tested in B (P < 0.05). (C) A gliadin-derived peptide conjugated with two NIP molecules NIP-LRMKLQPFPQPELPYPQPELPYLRMK(-NIP)L was used to form multimeric ICs in the presence of WT (filled symbols) or IHH-mutated (open symbols) NIPIgG. The Ags were presented by FcRn-positive DC (squares) or FcRn-negative, EBV transformed B cells (circles).
Multimeric FcRn Ligands Are Directed into Lysosomes in DC, Whereas Monomeric Ligands Are Not.
The studies described above show that not only does FcRn regulate Fc-dependent Ag presentation, but also that this regulation is observed only when the IgG–Ag complex is multimeric. This suggests that FcRn might direct the intracellular trafficking of its ligands differently depending on the valency of the bound ligands and thus determines whether Ag presentation is enhanced. To further test this hypothesis, we performed confocal studies to follow the trafficking of endogenously expressed FcRn in human monocyte-derived DC that was visualized by a biotinylated mouse anti-human FcRn monoclonal antibody. In the absence of ligands, FcRn in human DC was not observed to traffic to LAMP-positive lysosomes, in agreement with earlier results showing that FcRn avoids trafficking to lysosomes in endothelial cell lines (25) (Fig. 6A). Shortly after coincubation with WT OVA ICs, the Alexa647-labeled WT NIPIgG was taken up rapidly by DC and detected in membrane-proximal intracellular compartments, presumably early endosomes that colocalized with FcRn (Fig. 6B). After extended incubation periods of 25–50 min with these WT ICs, FcRn was observed to appear in LAMP1-positive lysosomes (Fig. 6D, arrowheads). Such a colocalization was not observed after a similar incubation of DC with IHH-mutated ICs that were unable to bind FcRn as confirmed by statistically significant lower colocalization of the FcRn and LAMP1 markers (Fig. 6E and Fig. S4) or after incubation with monomeric FcRn IgG–Ag complexes (Fig. 6C). These studies show that in the presence of multimeric, but not monomeric ICs or at baseline, FcRn and IgG are directed into lysosomes.
Fig. 6.
Trafficking of FcRn to LAMP1-positive lysosomes after IC stimulation in human monocyte derived DC. Human DC were incubated with Ags in HBSS, pH 7.4, at 37°C, fixed, permeabilized, and stained for LAMP1 encoded in green with rabbit polyclonal anti-LAMP1 and endogenously expressed FcRn encoded in red with biotinylated mouse IgG2b anti-human FcRn. (A) Medium. (B) Alexa647-conjugated FcRn-binding multimeric ICs encoded in blue, removed after 20 min by washing and further incubated in HBSS for 5 min. (C) Monomeric FcRn ligands, removed after 20 min and further incubated in HBSS for 30 min. (D) Alexa647-ICs encoded in blue, removed after 20 min and further incubated in HBSS for 30 min, and (E) Alexa647-conjugated FcRn nonbinding IHH-mutated ICs encoded in blue, removed after 20 min, and further incubated in HBSS for 30 min. Arrowheads in D indicate white areas of triple colocalized FcRn, IgG, and LAMP1, a phenomenon seen in ≈50% of cells investigated, and the arrow indicates yellow area of FcRn colocalized with LAMP1.
Hematopoietic Cell-Dependent Degradation of IgG in Multimeric Immune Complexes in Vivo.
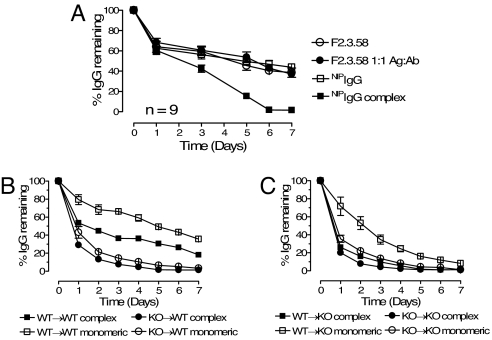
Recent studies have shown that FcRn in mouse hematopoietic cells participates in the protection of monomeric IgG from degradation (17). Based on our studies as shown above (Fig. 6), it would be predicted that multimeric IgG would not be protected from degradation as FcRn would mediate movement of multimeric IgG–Ag complexes into lysosomes. Therefore, to further probe whether the degradative fate of IgG in vivo depends on the IgG valency, we monitored the in vivo serum half-life of transferred IgG molecules found in different molecular complexes. Multimeric IgG-Ag ICs were formed by coincubation of WT NIPIgG and NIP-conjugated BSA (on average 15 NIP molecules per BSA protein), whereas monomeric IgG–Ag complexes were formed by coincubation of a mouse monoclonal anti-OVA IgG1, the F2.3.58 antibody (kindly provided by F. Fitch, University of Chicago, Chicago), together with OVA. We designed two ELISAs that were able to specifically measure these two antibodies without cross-reactivity (Fig. S5). Thus, we could cotransfer these two types of IgG-Ag ICs into the same WT Balb/C mouse and monitor the serum levels of NIPIgG and F2.3.58 antibodies independently. In a separate group of mice, we cotransferred similar amounts of Ag-free NIPIgG and F2.3.58 antibodies. The three monomeric IgG preparations, i.e., Ag-free NIPIgG and F2.3.58 antibodies, and F2.3.58 in monomeric complex with OVA, exhibited similar rates of degradation with a serum half-life of ≈5.5 days. In comparison, NIPIgG transferred as a part of large multimeric ICs displayed a markedly accelerated degradation with a serum half-life of ≈2.2 days (Fig. 7A).
Fig. 7.
IgG in multimeric ICs is cleared faster from the circulation than free IgG and monomeric IgG-Ag molecules with clearance dependent on FcRn expression in hematopoietic cells. (A) Two groups of WT BALB/c mice were injected i.v. with either WT NIPIgG (open square) mixed with monoclonal mouse anti-OVA F2.3.58 IgG1 (open circle), or mixtures of NIPIgG in multimeric ICs (NIPIgG + NIP-BSA, filled square) and F2.3.58 in monomeric complex (F2.3.58 + OVA, filled circle) in PBS. The remaining levels of both transferred antibodies were measured by specific ELISA and are shown as percentage of the starting serum IgG level in the same animal 2 h after injection. The NIPIgG complex group is significantly different from each of the other three groups (P < 0.01). (B) WT B6 Thy1.1 recipient mice were lethally irradiated and reconstituted with BM from WT (squares, WT→WT, n = 3) or FcRn-deficient Thy1.2 mice (circles, KO→W,T n = 3). Nine weeks after reconstitution, the mice were injected i.v. with NIPIgG + NIP-BSA ICs or monomeric F2.3.58-OVA complex. The serum levels of NIPIgG (filled symbols) and F2.3.58 (open symbols) were measured daily and are expressed as percentage of the starting level 2 h after injection. Pairwise comparisons with one-way ANOVA showed that all groups were statistically different from each other (P < 0.001 after Bonferroni correction) except complex and monomeric IgG in the KO→WT group (filled circle vs. open circle) that were not statistically different (P > 0.05). (C) Lethally irradiated FcRn-deficient mice were reconstituted with WT (squares, WT→KO, n = 5) or FcRn-deficient BM (circles, KO→KO n = 4). Nine weeks later, the mice received the same IgG transfer as in B. Monomeric IgG degradation in the WT→KO group (open square) was statistically different from each of the other three graphs (P < 0.01), whereas these three groups were not statistically different from each other. Error bars indicate standard error of the mean.
To further delineate the role of FcRn in the differential degradative fate of multimeric vs. monomeric IgG, we generated BM chimeric mice that were either WT or deficient for FcRn expression and followed the serum half-life of monomeric IgG (F2.3.58) and multimeric IgG (NIPIgG in ICs) in these mice. As expected, the serum decay of both antibodies were faster in FcRn-deficient recipient mice (Fig. 7C) compared with WT recipient mice (Fig. 7B), and both antibodies were also degraded faster in mice reconstituted with FcRn-deficient BM (circles) compared with mice transplanted with WT BM (squares, Fig. 7 B and C). These data show that cells of both myeloid and nonhematopoietic origins contribute to serum IgG protection, in agreement with recent published data (17). More interestingly, compared with the monomeric F2.3.58 antibody, the degradative rate of NIPIgG transferred as a part of large multimeric ICs was markedly increased in mice reconstituted with WT, FcRn-expressing BM, in both WT and FcRn-deficient recipients (squares, Fig. 7 B and C). In comparison, the degradation rates of NIPIgG in multimeric complex and monomeric F2.3.58 antibody were not significantly different within the groups of mice that were transplanted with FcRn-deficient BM (circles, Fig. 7 B and C). These data indicate that IgG present in multimeric ICs exhibits an in vivo fate that is consistent with active trafficking toward lysosomal degradation rather than being recycled by FcRn and protected from degradation, the default route for unbound IgG or IgG in monomeric complexes. In addition, this enhanced degradation of the immune complexes is FcRn-mediated and depends on hematopoietic cells, because a faster rate of degradation was observed in mice that contained WT FcRn BM compared with FcRn-deficient BM. Because myeloid cells are the only cell type within the hematopoietic lineage that express FcRn as shown here, these studies indicate that cells of the myeloid lineage are the major cell types responsible for the physiological consequences of the differential cellular trafficking observed.
Discussion
Since the initial report of FcRn expression in human monocytes, macrophages, and DC (16), the biological function of this expression has been unknown. Two recent reports show FcRn in hematopoietic cells may play a role in IgG protection (17), and polymorphonuclear neutrophils may facilitate IgG-mediated bacterial phagocytosis (26). In this report, we confirm FcRn expression in professional APC in both human and mouse and lack of expression in lymphocytes (16, 17). Furthermore, we now show that the expression of FcRn in DC is critically linked to the ability of these cells to present a particular class of Ags to T cells, i.e., multimeric ICs formed by multiple IgG molecules bound to a large globular protein Ag. Moreover, our studies suggest that the basis for this function is through the ability of FcRn to direct large multimeric ICs into lysosomes where Ag processing and loading take place. In contrast, neither multimeric ICs that are disabled in their ability to bind FcRn nor monomeric IgG–Ag complexes enter lysosomes and, consistent with this, do not promote Ag presentation relative to soluble Ag. More importantly, these differences specifically depended on FcRn function and were observed in the presence of an intact ability of both types of ICs to bind classical FcγR.
The majority of Ags gain access to DC via nonspecific pinocytosis and phagocytosis, whereas some are taken up more efficiently via receptor-mediated endocytosis such as through IgG via FcγRs (1). Indeed, we observed a slightly increased presentation of ICs compared with soluble Ags by FcRn-deficient DC in the murine in vitro assay, indicating a role for FcγR. However, we have identified FcRn as a structurally distinct Fc-binding receptor that is unique in the pH dependency of its binding as being critical for the efficient presentation of ICs by DCs in addition to FcγR. The expression level of FcRn on human DC surfaces is low and, under steady-state conditions, FcRn is observed to reside mostly intracellularly within early endosomes (ref. 16 and data not shown). Together with the fact that FcRn binds IgG poorly at neutral pH found in most extracellular spaces (6), it is unlikely that the cell surface of DC is the major site where the primary binding of IgG containing Ags to FcRn occurs. Rather, such Ags probably gain access to DC initially via either nonspecific pinocytosis, or more efficiently, through receptor-mediated uptake by binding, for example, to FcγR that are abundantly expressed on the cell surface of DC. Shortly after uptake, these IgG-containing Ags traffic to early endosomes where FcRn resides. During endosomal acidification, the pH within the endosomes decreases and approaches the range (pH <6.5) for efficient FcRn-Fc binding (27), whereas at the same time, FcγR-Fc binding is largely ineffective at pH <6.5 (data not shown). Therefore, it is probable that the initial FcRn-Fc binding first takes place within slightly acidified early endosomes.
Interestingly, after initial FcRn binding in early endosomes, the fate of the Fc-containing Ags seems to differ depending on the structure of the Ag complex as shown here. In the current study, functional and morphological evidence indicates that large multimeric ICs formed by multiple IgG molecules bound to an antigenic protein traffic to lysosomal compartments where further Ag processing and peptide loading onto MHC take place. In contrast, smaller monomeric complexes formed by one single antibody bound to each Ag molecule, such as those formed by NIPIgG and mono-NIP-conjugated peptides, were not observed to traffic to lysosomes and as a result were not more efficiently presented than non-FcRn-binding Ags with similar structures. That FcRn can traffic into lysosomes is in stark contrast to earlier studies on FcRn trafficking performed in the absence of ligand (IgG) or in the presence of monomeric IgG, wherein FcRn was reported to avoid trafficking to lysosomes (11, 25). Indeed, the specific evasion of lysosomes has been considered one of the hallmarks of FcRn trafficking by which FcRn prolongs the biological half-life of IgG. Our studies now indicate that this FcRn-mediated protection is applicable only for Ag-free IgG and IgG present in small monomeric IgG–Ag complexes. Once found as part of larger multimeric IgG–Ag complexes, those IgG molecules are shown to enter lysosomes. Consistent with this, large ICs are cleared much faster than either Ag-free IgG or monomeric IgG–Ag complexes from the circulation, and this accelerated degradation depends on the expression of FcRn in hematopoietic cells. Given the unique role of FcRn in IgG metabolism and our current finding that large IgG-Ag ICs bound to FcRn are being trafficked to lysosomes, we suggest that FcRn has a unique dual role in IgG metabolism. Whereas FcRn protects and prolongs the serum half-life of monomeric IgG under noninflammatory conditions, FcRn actively facilitates the degradation of presumably pathogenic multimeric IgG–Ag complexes by trafficking them to the lysosomes. When this takes place in a professional APC such as a DC, the lysosomal trafficking will also lead to the efficient presentation of Ag epitopes present in the ICs. As such, FcRn provides a crucial function in distinguishing between noninflammatory and inflammatory forms of IgG. Because microorganisms are characterized by repetitive Agic structures, FcRn may assist in the detection of their presence making FcRn of special importance to anti-microbial immunity. FcRn-deficient animals are indeed more sensitive to certain bacterial infections (28, 29).
Another aspect that deserves further study is the observation that FcRn nonbinding IHH-mutated ICs were not observed to enter lysosomes during the 50-min time period we studied. These results contrast with the notion that in the absence of FcRn-mediated protection, IgG enter the default lysosomal degradation pathway as has been observed in endothelial cells (25). One possible explanation is that DC, as a professional APC, handles FcRn-directed Ags as ICs that are destined for presentation differently from proteins such as monomeric IgG that are destined for simple lysosomal degradation. This notion is supported by a recent study showing that within the same DC, each phagosome can encounter different trafficking and processing pathways leading to different efficiency in presentation of the antigenic cargo depending on the presence of toll-like receptor ligands contained within each individual phagosome (30). Alternatively, IHH ICs could be rescued from lysosomal degradation by other Fc receptors because it has been reported that FcγRII can recycle ICs back to the cell surface (31).
The mechanism by which FcRn distinguishes and thus directs differential trafficking of multimeric vs. monomeric-bound ligands in a DC is unknown. One possibility is that the cross-linking of FcRn molecules by multiple Fc-binding sites on large multimeric ICs may elicit signals mediated by some as yet to be defined molecular partners that ultimately result in FcRn trafficking to lysosomes. Interestingly, one recent study on the trafficking patterns of IgG molecules with either two WT FcRn-binding Fc or a heterodimeric Fc with only one FcRn binding site has revealed that ligand valency affects transcytosis, recycling and intracellular trafficking mediated by FcRn (32). Another possibility that is not mutually exclusive is that because high-affinity FcγRI bind monomeric IgG, whereas low-affinity FcγRII can only bind multimeric ICs, FcRn may distinguish the structure of its bound ligands by recognizing the FcγR from which it receives its ligand in early endosomes.
It is interesting that these differences in behavior between monomeric and multimeric IgG–Ag complexes have been defined in a cell type that expresses classical FcγR raising the possibility that FcRn may be especially important or function differently in FcγR-bearing cell types (e.g., DC) vs. those that do not typically express FcγR (e.g., epithelial and endothelial cells). In total, these studies reveal a role for FcRn as an additional IgG receptor that is involved in Ag presentation drawing FcRn into a much broader role for immune function.
Materials and Methods
ICs and Ags.
The WT chimeric NIPIgG was described in ref. 19 and the IHH mutant was generated by site-directed mutagenesis as described in SI Text. For formation of ICs, see SI Text.
For Western blot analysis, ELISA, and in vitro T cell proliferation assays, see SI Text.
In Vivo T Cell Proliferation Assays.
CD4 T cells from DO11.10 mice bearing an OVA-reactive transgenic TCR were purified by sequential negative selection with Pan T cell Isolation Kit and CD8 microbeads (Miltenyi) and labeled with 5 μM CFDA-SE (Invitrogen). CFSE-labeled DO11.10 T cells (1 × 106) were injected i.v. in the tail veins of WT or FcRn-deficient Balb/C mice followed by s.c. injection of preformed NIPIgG (WT or IHH-mutated, 40 μg per injection) and NIP-OVA (15 μg per injection) ICs into hind footpads. Three to 5 days later, the draining popliteal lymph nodes were harvested and the CFSE intensity of DO11.10 T cells examined after gating on KJ1–26-positive cells (BD). For each sample, the theoretical number of mitosis undergone by each progenitor cell (M/P ratio) was calculated as M/P = [ΣCi − Σ(Ci/2i)]/[Σ(Ci/2i)], where Ci denotes the number of cell counts in each gated cell division.
Confocal Studies.
Human monocyte-derived DCs were adhered to poly-l-lysine-coated coverslips and incubated with preformed ICs of Alexa647-labeled (Invitrogen) WT or IHH-mutated NIPIgG (40 μg/ml) and NIP-OVA (20 μg/ml) in HBSS pH 7.4 for 20 min at 37°C. The cells were then washed, further incubated in HBSS for various times, and fixed with 4% paraformaldehyde, permeabilized with 0.2% saponin and blocked with 10% goat serum (Zymed). The cells were stained with rabbit LAMP1 polyclonal antibodies (Abcam), Alexa488-goat-anti-rabbit (Invitrogen), biotin-mouse IgG2b anti-human FcRn (ADM31), and streptavidin-Alexa568. The coverslips were mounted with ProLong Gold AntiFade (Invitrogen) and images captured on a Nikon TE2000-E inverted microscope coupled to a Perkin–Elmer spinning disk confocal unit.
For BM chimera and IgG half-life studies, see SI Text.
Statistics.
One-way ANOVA with Bonferroni posttest was used for pairwise comparison of all groups for Figs. 2, 5, and 7. The result in Fig. 3 was tested with Mann–Whitney test, and paired Wilcoxon test was used in Fig. 4. All statistical tests were run with Prism 4 software (GraphPad).
Supplementary Material
Acknowledgments.
We thank Jessica Wagner for assistance with confocal imaging, Helen Mah for HLA genotyping, and Eric de Muinck for assistance with ELISA. This work was supported by the Research Council of Norway (S.-W.Q.); the EMBIO program at the University of Oslo (J.T.A.); National Institutes of Health Grants DK53056, DK51362, and DK44319 (to R.S.B.); and the Harvard Digestive Disease Center (National Institutes of Health Grant DK34854).
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/cgi/content/full/0801717105/DCSupplemental.
References
- 1.Liu Y, et al. Regulated expression of FcγR in human dendritic cells controls cross-presentation of antigen-antibody complexes. J Immunol. 2006;177:8440–8447. doi: 10.4049/jimmunol.177.12.8440. [DOI] [PubMed] [Google Scholar]
- 2.Regnault A, et al. Fcγ receptor-mediated induction of dendritic cell maturation and major histocompatibility complex class I-restricted antigen presentation after immune complex internalization. J Exp Med. 1999;189:371–380. doi: 10.1084/jem.189.2.371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ravetch JV, Bolland S. IgG Fc receptors. Annu Rev Immunol. 2001;19:275–290. doi: 10.1146/annurev.immunol.19.1.275. [DOI] [PubMed] [Google Scholar]
- 4.Hirano M, et al. IgEb immune complexes activate macrophages through FcγRIV binding. Nat Immunol. 2007;8:762–771. doi: 10.1038/ni1477. [DOI] [PubMed] [Google Scholar]
- 5.Simister NE, Mostov KE. An Fc receptor structurally related to MHC class I antigens. Nature. 1989;337:184–187. doi: 10.1038/337184a0. [DOI] [PubMed] [Google Scholar]
- 6.Rodewald R. pH-dependent binding of immunoglobulins to intestinal cells of the neonatal rat. J Cell Biol. 1976;71:666–669. doi: 10.1083/jcb.71.2.666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chaudhury C, et al. The major histocompatibility complex-related Fc receptor for IgG (FcRn) binds albumin and prolongs its lifespan. J Exp Med. 2003;197:315–322. doi: 10.1084/jem.20021829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Wines BD, Powell MS, Parren PWHI, Barnes N, Hogarth PM. The IgG Fc contains distinct Fc receptor (FcR) binding sites: The leukocyte receptors FcγRI and FcγRIIa bind to a region in the Fc distinct from that recognized by neonatal FcR and protein A. J Immunol. 2000;164:5313–5318. doi: 10.4049/jimmunol.164.10.5313. [DOI] [PubMed] [Google Scholar]
- 9.Shields RL, et al. High resolution mapping of the binding site on human IgG1 for FcγRI, FcγRII, FcγRIII, and FcRn and design of IgG1 variants with improved binding to the FcγR. J Biol Chem. 2001;276:6591–6604. doi: 10.1074/jbc.M009483200. [DOI] [PubMed] [Google Scholar]
- 10.Israel EJ, Patel VK, Taylor SF, Marshak-Rothstein A, Simister NE. Requirement for a β2-microglobulin-associated Fc receptor for acquisition of maternal IgG by fetal and neonatal mice. J Immunol. 1995;154:6246–6251. [PubMed] [Google Scholar]
- 11.Junghans RP, Anderson CL. The protection receptor for IgG catabolism is the β2-microglobulin-containing neonatal intestinal transport receptor. Proc Natl Acad Sci USA. 1996;93:5512–5516. doi: 10.1073/pnas.93.11.5512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Yoshida M, et al. Human neonatal Fc receptor mediates transport of IgG into luminal secretions for delivery of antigens to mucosal dendritic cells. Immunity. 2004;20:769–783. doi: 10.1016/j.immuni.2004.05.007. [DOI] [PubMed] [Google Scholar]
- 13.Israel EJ, et al. Expression of the neonatal Fc receptor, FcRn, on human intestinal epithelial cells. Immunology. 1997;92:69–74. doi: 10.1046/j.1365-2567.1997.00326.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Spiekermann GM, et al. Receptor-mediated immunoglobulin G transport across mucosal barriers in adult life: functional expression of FcRn in the mammalian lung. J Exp Med. 2002;196:303–310. doi: 10.1084/jem.20020400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Haymann J-P, et al. Characterization and localization of the neonatal Fc receptor in adult human kidney. J Am Soc Nephrol. 2000;11:632–639. doi: 10.1681/ASN.V114632. [DOI] [PubMed] [Google Scholar]
- 16.Zhu X, et al. MHC class I-related neonatal Fc receptor for IgG is functionally expressed in monocytes, intestinal macrophages, and dendritic cells. J Immunol. 2001;166:3266–3276. doi: 10.4049/jimmunol.166.5.3266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Akilesh S, Christianson GJ, Roopenian DC, Shaw AS. Neonatal FcR Expression in bone marrow-derived cells functions to protect serum IgG from catabolism. J Immunol. 2007;179:4580–4588. doi: 10.4049/jimmunol.179.7.4580. [DOI] [PubMed] [Google Scholar]
- 18.Ober RJ, Radu CG, Ghetie V, Ward ES. Differences in promiscuity for antibody-FcRn interactions across species: implications for therapeutic antibodies. Int Immunol. 2001;13:1551–1559. doi: 10.1093/intimm/13.12.1551. [DOI] [PubMed] [Google Scholar]
- 19.Claypool SM, et al. Bidirectional transepithelial IgG transport by a strongly polarized basolateral membrane Fcγ-receptor. Mol Biol Cell. 2004;15:1746–1759. doi: 10.1091/mbc.E03-11-0832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kim JK, Tsen MF, Ghetie V, Ward ES. Localization of the site of the murine IgG1 molecule that is involved in binding to the murine intestinal Fc receptor. Eur J Immunol. 1994;24:2429–2434. doi: 10.1002/eji.1830241025. [DOI] [PubMed] [Google Scholar]
- 21.Medesan C, Matesoi D, Radu C, Ghetie V, Ward ES. Delineation of the amino acid residues involved in transcytosis and catabolism of mouse IgG1. J Immunol. 1997;158:2211–2217. [PubMed] [Google Scholar]
- 22.Kim JK, et al. Mapping the site on human IgG for binding of the MHC class I-related receptor, FcRn. Eur J Immunol. 1999;29:2819–2825. doi: 10.1002/(SICI)1521-4141(199909)29:09<2819::AID-IMMU2819>3.0.CO;2-6. [DOI] [PubMed] [Google Scholar]
- 23.Wells AD, Gudmundsdottir H, Turka LA. Following the fate of individual T cells throughout activation and clonal expansion. Signals from T cell receptor and CD28 differentially regulate the induction and duration of a proliferative response. J Clin Invest. 1997;100:3173–3183. doi: 10.1172/JCI119873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Qiao SW, et al. Refining the rules of gliadin T cell epitope binding to the disease-associated DQ2 molecule in celiac disease: Importance of proline spacing and glutamine deamidation. J Immunol. 2005;175:254–261. doi: 10.4049/jimmunol.175.1.254. [DOI] [PubMed] [Google Scholar]
- 25.Ober RJ, Martinez C, Vaccaro C, Zhou J, Ward ES. Visualizing the site and dynamics of IgG salvage by the MHC class I-related receptor, FcRn. J Immunol. 2004;172:2021–2029. doi: 10.4049/jimmunol.172.4.2021. [DOI] [PubMed] [Google Scholar]
- 26.Vidarsson G, et al. FcRn: an IgG receptor on phagocytes with a novel role in phagocytosis. Blood. 2006;108:3573–3579. doi: 10.1182/blood-2006-05-024539. [DOI] [PubMed] [Google Scholar]
- 27.Roopenian DC, Akilesh S. FcRn: The neonatal Fc receptor comes of age. Nat Rev Immunol. 2007;7:715–725. doi: 10.1038/nri2155. [DOI] [PubMed] [Google Scholar]
- 28.Yoshida M, et al. Neonatal Fc receptor for IgG regulates mucosal immune responses to luminal bacteria. J Clin Invest. 2006;116:2142–2152. doi: 10.1172/JCI27821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Crowley H, Alroy J, Sproule TJ, Roopenian D, Huber BT. The MHC class I-related FcRn ameliorates murine Lyme arthritis. Int Immunol. 2006;18:409–414. doi: 10.1093/intimm/dxh380. [DOI] [PubMed] [Google Scholar]
- 30.Blander JM, Medzhitov R. Toll-dependent selection of microbial antigens for presentation by dendritic cells. Nature. 2006;440:808–812. doi: 10.1038/nature04596. [DOI] [PubMed] [Google Scholar]
- 31.Bergtold A, Desai DD, Gavhane A, Clynes R. Cell surface recycling of internalized antigen permits dendritic cell priming of B cells. Immunity. 2005;23:503–514. doi: 10.1016/j.immuni.2005.09.013. [DOI] [PubMed] [Google Scholar]
- 32.Tesar DB, Tiangco NE, Bjorkman PJ. Ligand valency affects transcytosis, recycling and intracellular trafficking mediated by the neonatal Fc receptor. Traffic. 2006;7:1127–1142. doi: 10.1111/j.1600-0854.2006.00457.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.