Abstract
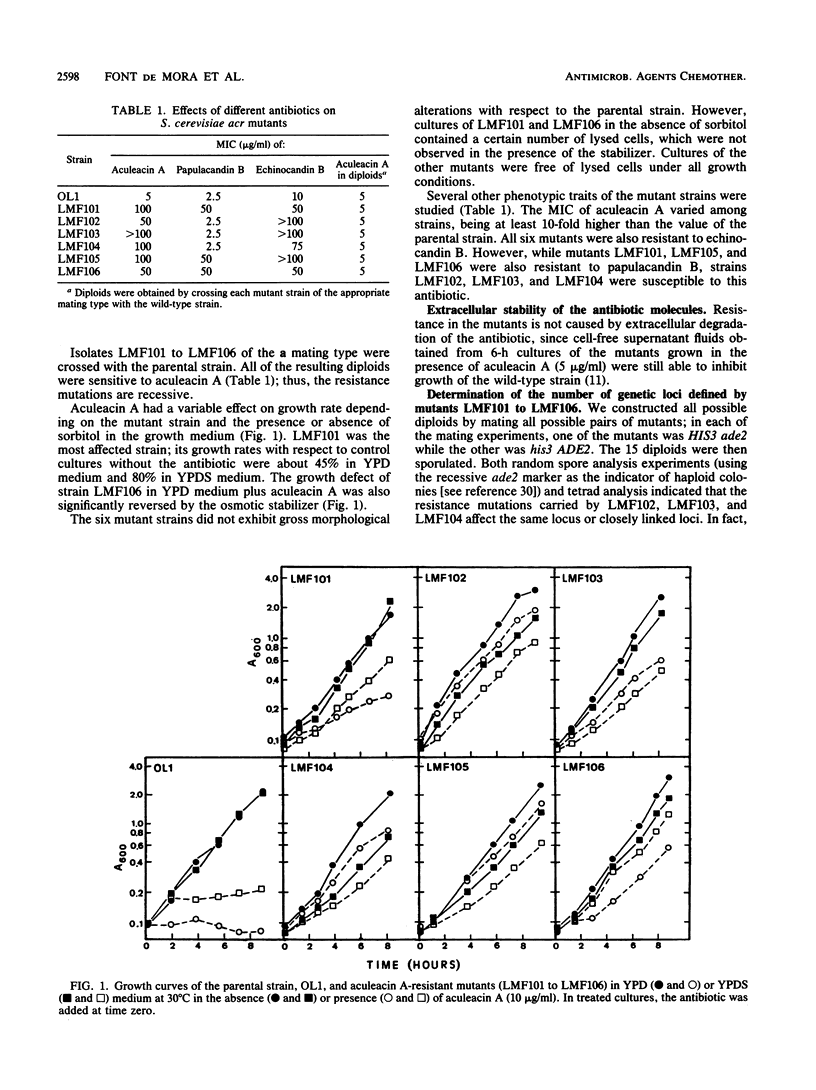
Aculeacin A is a lipopeptide that inhibits beta-glucan synthesis in yeasts. A number of Saccharomyces cerevisiae mutants resistant to this antibiotic were isolated, and four loci (ACR1, ACR2, ACR3, and ACR4) whose products are involved in the sensitivity to aculeacin A of yeast cells were defined. Mutants containing mutations in the four loci were also resistant to echinocandin B, another member of this lipopeptide family of antibiotics. In contrast, acr1, acr3, and acr4 mutants were resistant to papulacandin B (an antibiotic containing a disaccharide linked to two fatty acid chains that also inhibits beta-glucan synthesis), but acr2 mutants were susceptible to this antibiotic. This result defines common and specific steps in the entry and action of aculeacin A and papulacandin B. The analysis of double mutants revealed an epistatic effect of the acr2 mutation on the other three mutations. Cell walls of the four different mutants did not show significant alterations in composition with respect to the parental strain, and in vitro glucan synthase activity was also unaffected. However, cell surface hydrophobicity in three of the mutants was considerably decreased with respect to the parental strain.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Baguley B. C., Römmele G., Gruner J., Wehrli W. Papulacandin B: an inhibitor of glucan synthesis in yeast spheroplasts. Eur J Biochem. 1979 Jul;97(2):345–351. doi: 10.1111/j.1432-1033.1979.tb13120.x. [DOI] [PubMed] [Google Scholar]
- Boy-Marcotte E., Jacquet M. A Dictyostelium discoideum DNA fragment complements a Saccharomyces cerevisiae ura3 mutant. Gene. 1982 Dec;20(3):433–439. doi: 10.1016/0378-1119(82)90212-8. [DOI] [PubMed] [Google Scholar]
- Bulawa C. E., Osmond B. C. Chitin synthase I and chitin synthase II are not required for chitin synthesis in vivo in Saccharomyces cerevisiae. Proc Natl Acad Sci U S A. 1990 Oct;87(19):7424–7428. doi: 10.1073/pnas.87.19.7424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bush D. A., Horisberger M., Horman I., Wursch P. The wall structure of Schizosaccharomyces pombe. J Gen Microbiol. 1974 Mar;81(1):199–206. doi: 10.1099/00221287-81-1-199. [DOI] [PubMed] [Google Scholar]
- Debono M., Abbott B. J., Turner J. R., Howard L. C., Gordee R. S., Hunt A. S., Barnhart M., Molloy R. M., Willard K. E., Fukuda D. Synthesis and evaluation of LY121019, a member of a series of semisynthetic analogues of the antifungal lipopeptide echinocandin B. Ann N Y Acad Sci. 1988;544:152–167. doi: 10.1111/j.1749-6632.1988.tb40398.x. [DOI] [PubMed] [Google Scholar]
- Emr S. D., Schekman R., Flessel M. C., Thorner J. An MF alpha 1-SUC2 (alpha-factor-invertase) gene fusion for study of protein localization and gene expression in yeast. Proc Natl Acad Sci U S A. 1983 Dec;80(23):7080–7084. doi: 10.1073/pnas.80.23.7080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gordee R. S., Zeckner D. J., Ellis L. F., Thakkar A. L., Howard L. C. In vitro and in vivo anti-Candida activity and toxicology of LY121019. J Antibiot (Tokyo) 1984 Sep;37(9):1054–1065. doi: 10.7164/antibiotics.37.1054. [DOI] [PubMed] [Google Scholar]
- Hazen K. C., Lay J. G., Hazen B. W., Fu R. C., Murthy S. Partial biochemical characterization of cell surface hydrophobicity and hydrophilicity of Candida albicans. Infect Immun. 1990 Nov;58(11):3469–3476. doi: 10.1128/iai.58.11.3469-3476.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hazen K. C. Participation of yeast cell surface hydrophobicity in adherence of Candida albicans to human epithelial cells. Infect Immun. 1989 Jul;57(7):1894–1900. doi: 10.1128/iai.57.7.1894-1900.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kennedy M. J. Adhesion and association mechanisms of Candida albicans. Curr Top Med Mycol. 1988;2:73–169. doi: 10.1007/978-1-4612-3730-3_4. [DOI] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Lopez-Ribot J. L., Casanova M., Martinez J. P., Sentandreu R. Characterization of cell wall proteins of yeast and hydrophobic mycelial cells of Candida albicans. Infect Immun. 1991 Jul;59(7):2324–2332. doi: 10.1128/iai.59.7.2324-2332.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mehta R. J., Nash C. H., Grappel S. F., Actor P. Aculeacin A resistant mutants of Candida albicans. J Antibiot (Tokyo) 1982 Jun;35(6):707–711. doi: 10.7164/antibiotics.35.707. [DOI] [PubMed] [Google Scholar]
- Mizoguchi J., Saito T., Mizuno K., Hayano K. On the mode of action of a new antifungal antibiotic, aculeacin A: inhibition of cell wall synthesis in Saccharomyces cerevisiae. J Antibiot (Tokyo) 1977 Apr;30(4):308–313. doi: 10.7164/antibiotics.30.308. [DOI] [PubMed] [Google Scholar]
- Mizuno K., Yagi A., Satoi S., Takada M., Hayashi M. Studies on aculeacin. I. Isolation and characterization of aculeacin A. J Antibiot (Tokyo) 1977 Apr;30(4):297–302. doi: 10.7164/antibiotics.30.297. [DOI] [PubMed] [Google Scholar]
- Murgui A., Elorza M. V., Sentandreu R. Effect of papulacandin B and calcofluor white on the incorporation of mannoproteins in the wall of Candida albicans blastospores. Biochim Biophys Acta. 1985 Aug 16;841(2):215–222. doi: 10.1016/0304-4165(85)90024-8. [DOI] [PubMed] [Google Scholar]
- Orlean P. A. (1,3)-beta-D-Glucan synthase from budding and filamentous cultures of the dimorphic fungus Candida albicans. Eur J Biochem. 1982 Oct;127(2):397–403. doi: 10.1111/j.1432-1033.1982.tb06885.x. [DOI] [PubMed] [Google Scholar]
- Orlean P. Two chitin synthases in Saccharomyces cerevisiae. J Biol Chem. 1987 Apr 25;262(12):5732–5739. [PubMed] [Google Scholar]
- Ribas J. C., Roncero C., Rico H., Durán A. Characterization of a Schizosaccharomyces pombe morphological mutant altered in the galactomannan content. FEMS Microbiol Lett. 1991 Apr 15;63(2-3):263–267. doi: 10.1016/0378-1097(91)90096-s. [DOI] [PubMed] [Google Scholar]
- Sawistowska-Schröder E. T., Kerridge D., Perry H. Echinocandin inhibition of 1,3-beta-D-glucan synthase from Candida albicans. FEBS Lett. 1984 Jul 23;173(1):134–138. doi: 10.1016/0014-5793(84)81032-7. [DOI] [PubMed] [Google Scholar]
- Shematek E. M., Braatz J. A., Cabib E. Biosynthesis of the yeast cell wall. I. Preparation and properties of beta-(1 leads to 3)glucan synthetase. J Biol Chem. 1980 Feb 10;255(3):888–894. [PubMed] [Google Scholar]
- Silverman S. J., Sburlati A., Slater M. L., Cabib E. Chitin synthase 2 is essential for septum formation and cell division in Saccharomyces cerevisiae. Proc Natl Acad Sci U S A. 1988 Jul;85(13):4735–4739. doi: 10.1073/pnas.85.13.4735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Szaniszlo P. J., Kang M. S., Cabib E. Stimulation of beta(1----3)glucan synthetase of various fungi by nucleoside triphosphates: generalized regulatory mechanism for cell wall biosynthesis. J Bacteriol. 1985 Mar;161(3):1188–1194. doi: 10.1128/jb.161.3.1188-1194.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taft C. S., Stark T., Selitrennikoff C. P. Cilofungin (LY121019) inhibits Candida albicans (1-3)-beta-D-glucan synthase activity. Antimicrob Agents Chemother. 1988 Dec;32(12):1901–1903. doi: 10.1128/aac.32.12.1901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tang J., Parr T. R., Jr W-1 solubilization and kinetics of inhibition by cilofungin of Candida albicans (1,3)-beta-D-glucan synthase. Antimicrob Agents Chemother. 1991 Jan;35(1):99–103. doi: 10.1128/aac.35.1.99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Valentín E., Herrero E., Sentandreu R. Incorporation of mannoproteins into the walls of aculeacin A-treated yeast cells. Arch Microbiol. 1986 Dec;146(3):214–220. doi: 10.1007/BF00403219. [DOI] [PubMed] [Google Scholar]
- Yamaguchi H., Hiratani T., Baba M., Osumi M. Effect of aculeacin A, a wall-active antibiotic, on synthesis of the yeast cell wall. Microbiol Immunol. 1985;29(7):609–623. doi: 10.1111/j.1348-0421.1985.tb00865.x. [DOI] [PubMed] [Google Scholar]
- Yamaguchi H., Hiratani T., Iwata K., Yamamoto Y. Studies on the mechanism of antifungal action of aculeacin A. J Antibiot (Tokyo) 1982 Feb;35(2):210–219. doi: 10.7164/antibiotics.35.210. [DOI] [PubMed] [Google Scholar]



