Abstract
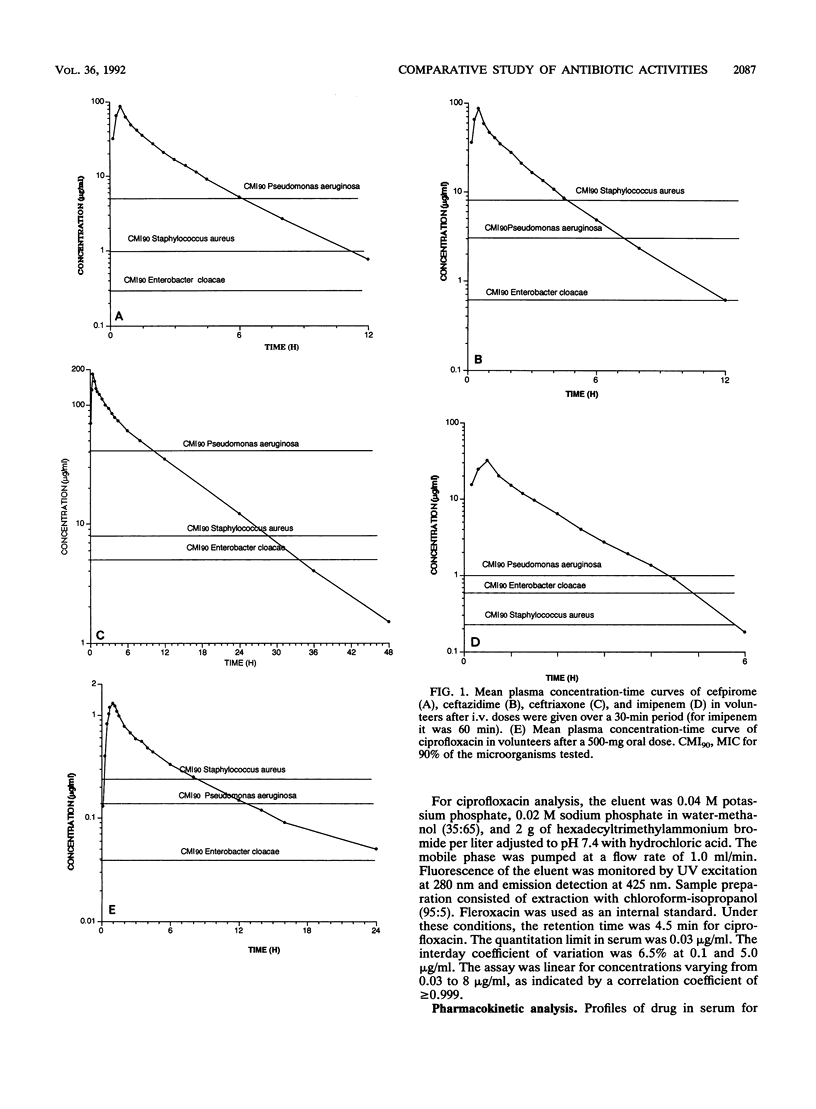
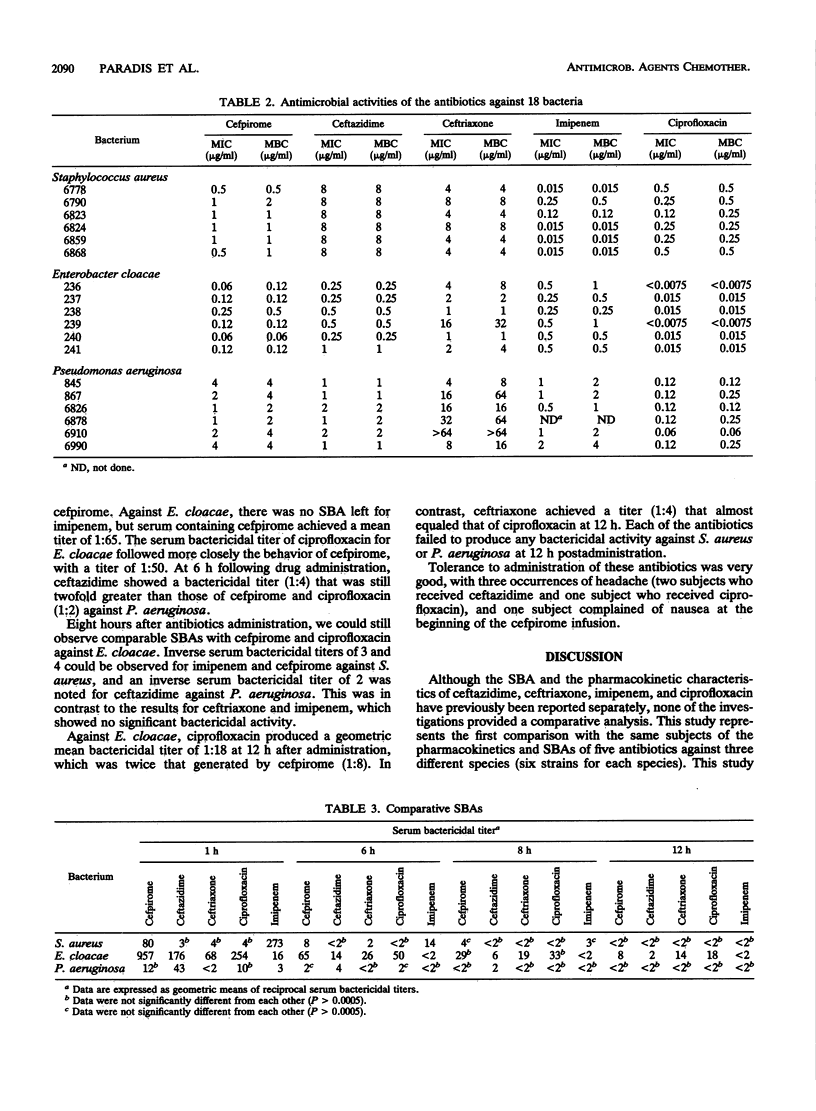
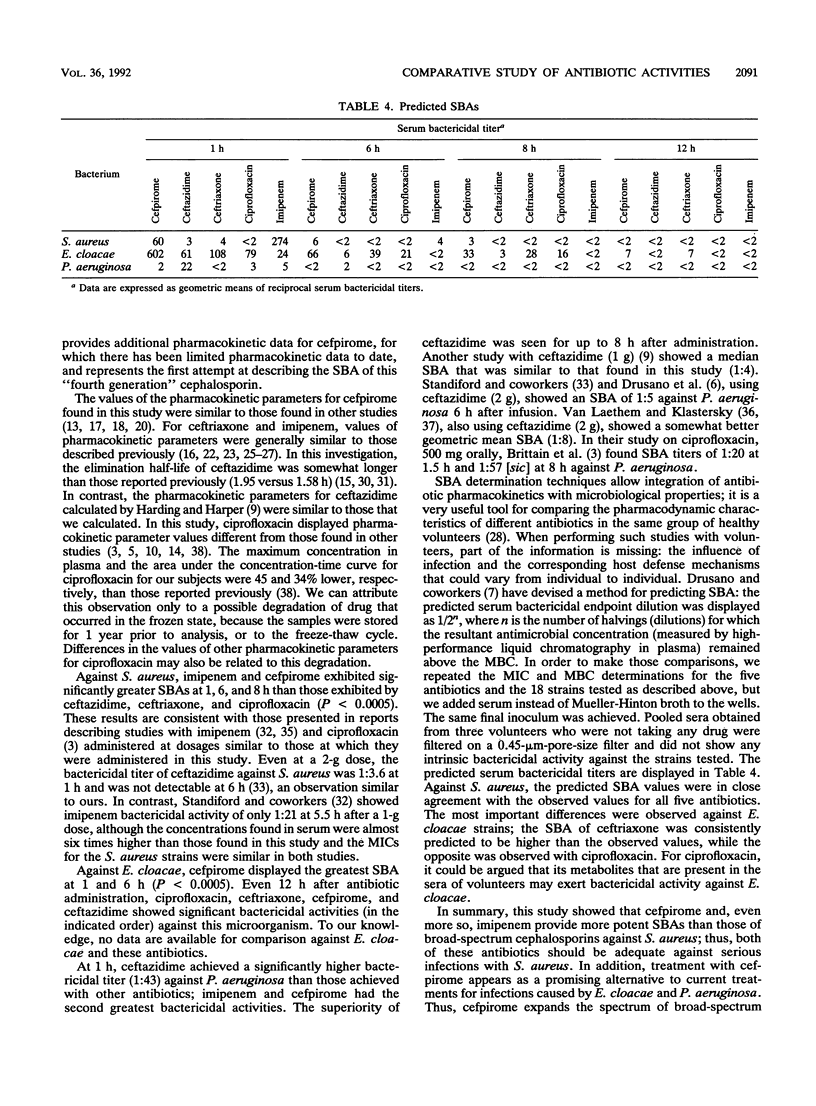
We compared the pharmacokinetics and the serum bactericidal activities of cefpirome, ceftazidime, ceftriaxone, imipenem, and ciprofloxacin. Fifteen healthy volunteers received 1 g of cefpirome, ceftazidime, and ceftriaxone intravenously, 500 mg of imipenem-cilastatin intravenously, and 500 mg of ciprofloxacin orally. High-performance liquid chromatographic assays were used to quantitate unchanged antibiotic in plasma and urine. Serum bactericidal activities were determined against six clinical isolates each of Staphylococcus aureus, Enterobacter cloacae, and Pseudomonas aeruginosa by using a modified microdilution method of Reller and Stratton (L. B. Reller and C. W. Stratton, J. Infect. Dis. 136:196-204, 1977). Overall, cefpirome exhibited pharmacokinetics similar to those of ceftazidime: half-life (t1/2), 1.95 h; concentration at 1 h (C1h), 47 to 49 micrograms/ml for both antibiotics. Ceftriaxone displayed the longest t1/2 (7.65 h) and the highest C1h (137.8 micrograms/ml), while we observed the shortest t1/2 (1.05 h) and the lowest C1h (19.85 micrograms/ml) with imipenem. At 1 h, cefpirome and, even more so, imipenem showed significantly better serum bactericidal activities against S. aureus (1:273 and 1:80) than did the other antibiotics (P less than 0.0005; analysis of variance with randomized block design and Bonferroni correction). Against E. cloacae, we observed the highest serum bactericidal titers at 1 h with cefpirome, and this superiority vis-à-vis the other antibiotics tested was maintained for up to 8 h after dosing. Ceftazidime remained the most active agent tested against P. aeruginosa (serum bactericidal activity titers, 1:43 at 1 h) up to 8 h. In summary, the study showed that cefpirome and imipenem provide more potent serum bactericidal activities than do broad-spectrum cephalosporins against S. aureus; thus, both of these antibiotics should be adequate against serious S. aureus infections. In addition, cefpirome appears to be a promising alternative for treatment of infections caused by E. cloacae and P. aeruginosa.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bauernfeind A. Susceptibility of gram-positive aerobic cocci to the new cephalosporin HR 810. Eur J Clin Microbiol. 1983 Aug;2(4):354–355. doi: 10.1007/BF02019468. [DOI] [PubMed] [Google Scholar]
- Bertram M. A., Bruckner D. A., Young L. S. In vitro activity of HR 810, a new cephalosporin. Antimicrob Agents Chemother. 1984 Aug;26(2):277–279. doi: 10.1128/aac.26.2.277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brittain D. C., Scully B. E., McElrath M. J., Steinman R., Labthavikul P., Neu H. C. The pharmacokinetics and serum and urine bactericidal activity of ciprofloxacin. J Clin Pharmacol. 1985 Mar;25(2):82–88. doi: 10.1002/j.1552-4604.1985.tb02806.x. [DOI] [PubMed] [Google Scholar]
- Davis R. L., Koup J. R., Williams-Warren J., Weber A., Smith A. L. Pharmacokinetics of three oral formulations of ciprofloxacin. Antimicrob Agents Chemother. 1985 Jul;28(1):74–77. doi: 10.1128/aac.28.1.74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drusano G. L., Standiford H. C., Fitzpatrick B., Leslie J., Tangtatsawasdi P., Ryan P., Tatem B., Moody M. R., Schimpff S. C. Comparison of the pharmacokinetics of ceftazidime and moxalactam and their microbiological correlates in volunteers. Antimicrob Agents Chemother. 1984 Sep;26(3):388–393. doi: 10.1128/aac.26.3.388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drusano G., Standiford H., Ryan P., McNamee W., Tatem B., Schimpff S. Correlation of predicted serum bactericidal activities and values measured in volunteers. Eur J Clin Microbiol. 1986 Feb;5(1):88–92. doi: 10.1007/BF02013475. [DOI] [PubMed] [Google Scholar]
- Gravallese D. A., Musson D. G., Pauliukonis L. T., Bayne W. F. Determination of imipenem (N-formimidoyl thienamycin) in human plasma and urine by high-performance liquid chromatography, comparison with microbiological methodology and stability. J Chromatogr. 1984 Sep 14;310(1):71–84. doi: 10.1016/0378-4347(84)80069-9. [DOI] [PubMed] [Google Scholar]
- Harding S. M., Harper P. B. The pharmacokinetic behaviour of ceftazidime in man and the relationship between serum levels and the in vitro susceptibility of clinical isolates. Infection. 1983;11 (Suppl 1):S49–S53. doi: 10.1007/BF01641107. [DOI] [PubMed] [Google Scholar]
- Höffler D., Dalhoff A., Gau W., Beermann D., Michl A. Dose- and sex-independent disposition of ciprofloxacin. Eur J Clin Microbiol. 1984 Aug;3(4):363–366. doi: 10.1007/BF01977496. [DOI] [PubMed] [Google Scholar]
- Jones R. N., Thornsberry C., Barry A. L. In vitro evaluation of HR810, a new wide-spectrum aminothiazolyl alpha-methoxyimino cephalosporin. Antimicrob Agents Chemother. 1984 Jun;25(6):710–718. doi: 10.1128/aac.25.6.710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kavi J., Andrews J. M., Ashby J. P., Hillman G., Wise R. Pharmacokinetics and tissue penetration of cefpirome, a new cephalosporin. J Antimicrob Chemother. 1988 Dec;22(6):911–916. doi: 10.1093/jac/22.6.911. [DOI] [PubMed] [Google Scholar]
- LeBel M., Vallée F., Bergeron M. G. Tissue penetration of ciprofloxacin after single and multiple doses. Antimicrob Agents Chemother. 1986 Mar;29(3):501–505. doi: 10.1128/aac.29.3.501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leroy A., Leguy F., Borsa F., Spencer G. R., Fillastre J. P., Humbert G. Pharmacokinetics of ceftazidime in normal and uremic subjects. Antimicrob Agents Chemother. 1984 May;25(5):638–642. doi: 10.1128/aac.25.5.638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luderer J. R., Patel I. H., Durkin J., Schneck D. W. Age and ceftriaxone kinetics. Clin Pharmacol Ther. 1984 Jan;35(1):19–25. doi: 10.1038/clpt.1984.3. [DOI] [PubMed] [Google Scholar]
- Maass L., Malerczyk V., Verho M., Hajdú P., Seeger K., Klesel N. Dose linearity testing of intravenous cefpirome (HR 810), a novel cephalosporin derivate. Infection. 1987 May-Jun;15(3):202–206. doi: 10.1007/BF01646051. [DOI] [PubMed] [Google Scholar]
- Maass L., Malerczyk V., Verho M. Pharmacokinetics of cefpirome (HR 810), a new cephalosporin derivative administered intramuscularly and intravenously to healthy volunteers. Infection. 1987 May-Jun;15(3):207–210. doi: 10.1007/BF01646052. [DOI] [PubMed] [Google Scholar]
- Machka K., Braveny I. In vitro activity of HR 810, a new broad-spectrum cephalosporin. Eur J Clin Microbiol. 1983 Aug;2(4):345–349. doi: 10.1007/BF02019465. [DOI] [PubMed] [Google Scholar]
- Malerczyk V., Maass L., Verho M., Hajdú P., Klesel N., Rangoonwala R. Single and multiple dose pharmacokinetics of intravenous cefpirome (HR 810), a novel cephalosporin derivative. Infection. 1987 May-Jun;15(3):211–214. doi: 10.1007/BF01646053. [DOI] [PubMed] [Google Scholar]
- Norrby S. R., Björnegård B., Ferber F., Jones K. H. Pharmacokinetics of imipenem in healthy volunteers. J Antimicrob Chemother. 1983 Dec;12 (Suppl 500):109–124. doi: 10.1093/jac/12.suppl_d.109. [DOI] [PubMed] [Google Scholar]
- Patel I. H., Chen S., Parsonnet M., Hackman M. R., Brooks M. A., Konikoff J., Kaplan S. A. Pharmacokinetics of ceftriaxone in humans. Antimicrob Agents Chemother. 1981 Nov;20(5):634–641. doi: 10.1128/aac.20.5.634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reller L. B., Stratton C. W. Serum dilution test for bactericidal activity. II. Standardization and correlation with antimicrobial assays and susceptibility tests. J Infect Dis. 1977 Aug;136(2):196–204. doi: 10.1093/infdis/136.2.196. [DOI] [PubMed] [Google Scholar]
- Rolinson G. N., Sutherland R. The binding of antibiotics to serum proteins. Br J Pharmacol Chemother. 1965 Dec;25(3):638–650. doi: 10.1111/j.1476-5381.1965.tb01788.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schimpff S. C., Drusano G. L., Standiford H. C. Serum bactericidal test in volunteers--a review. Eur J Clin Microbiol. 1986 Feb;5(1):71–78. doi: 10.1007/BF02013473. [DOI] [PubMed] [Google Scholar]
- Scully B. E., Fu K. P., Neu H. C. Pharmacokinetics of ceftriaxone after intravenous infusion and intramuscular injection. Am J Med. 1984 Oct 19;77(4C):112–116. [PubMed] [Google Scholar]
- Seibert G., Limbert M., Winkler I., Dick T. The Antibacterial activity in vitro and beta-lactamase stability of the new cephalosporin HR 810 in comparison with five other cephalosporins and two aminoglycosides. Infection. 1983 Sep-Oct;11(5):275–279. doi: 10.1007/BF01641262. [DOI] [PubMed] [Google Scholar]
- Sommers D. K., Walters L., Van Wyk M., Harding S. M., Paton A. M., Ayrton J. Pharmacokinetics of ceftazidime in male and female volunteers. Antimicrob Agents Chemother. 1983 Jun;23(6):892–896. doi: 10.1128/aac.23.6.892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Standiford H. C., Drusano G. L., Bustamante C. I., Rivera G., Forrest A., Tatem B., Leslie J., Moody M. Imipenem coadministered with cilastatin compared with moxalactam: integration of serum pharmacokinetics and microbiologic activity following single-dose administration to normal volunteers. Antimicrob Agents Chemother. 1986 Mar;29(3):412–417. doi: 10.1128/aac.29.3.412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Standiford H. C., Drusano G. L., Fitzpatrick B., Tatem B., Schimpff S. C. Bactericidal activity of ceftazidime in serum compared with that of ticarcillin combined with amikacin. Antimicrob Agents Chemother. 1984 Sep;26(3):339–342. doi: 10.1128/aac.26.3.339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stratton C. W., Weinstein M. P., Reller L. B. Correlation of serum bactericidal activity with antimicrobial agent level and minimal bactericidal concentration. J Infect Dis. 1982 Feb;145(2):160–168. doi: 10.1093/infdis/145.2.160. [DOI] [PubMed] [Google Scholar]
- Van Laethem Y., Klastersky J. Serum bactericidal activity of mezlocillin, ceftazidime, mezlocillin/ceftazidime and mezlocillin/amikacin against Klebsiella pneumoniae and Pseudomonas aeruginosa. Eur J Clin Microbiol. 1986 Feb;5(1):110–114. doi: 10.1007/BF02013479. [DOI] [PubMed] [Google Scholar]
- Van Laethem Y., Lagast H., Klastersky J. Serum bactericidal activity of ceftazidime and cefoperazone alone or in combination with amikacin against Pseudomonas aeruginosa and Klebsiella pneumoniae. Antimicrob Agents Chemother. 1983 Mar;23(3):435–439. doi: 10.1128/aac.23.3.435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van der Auwera P., Van Laethem Y., Klastersky J. Bactericidal activity and killing rate of serum against gram-positive cocci in volunteers receiving imipenem, oxacillin, vancomycin or ampicillin plus gentamicin. J Antimicrob Chemother. 1987 Aug;20(2):239–249. doi: 10.1093/jac/20.2.239. [DOI] [PubMed] [Google Scholar]


