Abstract
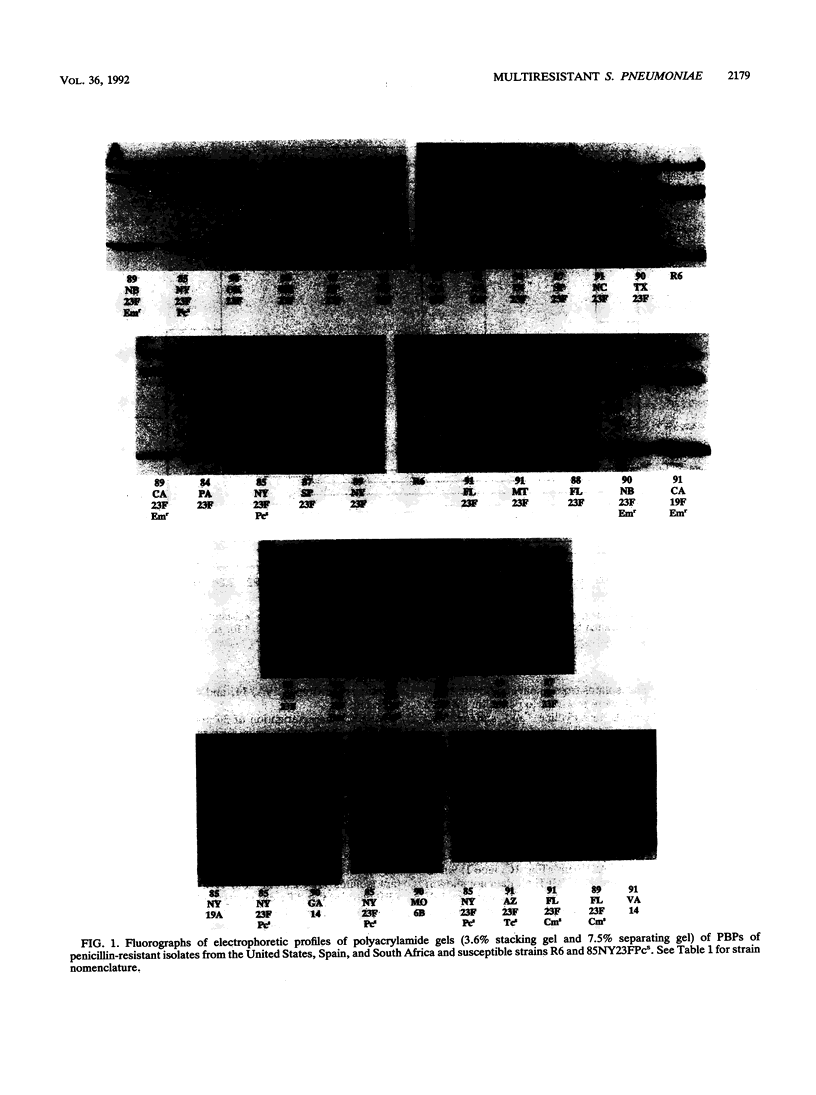
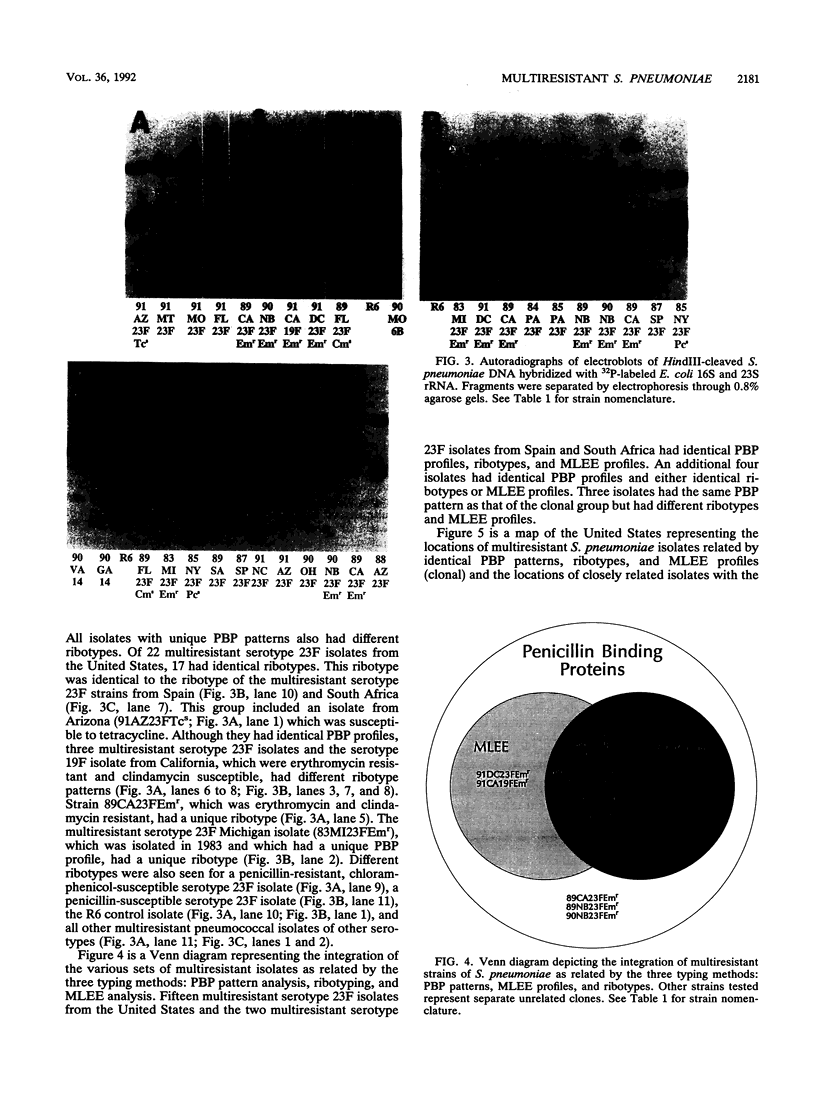
Streptococcus pneumoniae isolates resistant to penicillin, chloramphenicol, tetracycline and sulfamethoxazole-trimethroprim are being recovered with increasing frequency in the United States. We analyzed the penicillin-binding proteins (PBPs), multilocus enzyme electrophoresis (MLEE) genotypes, and ribotypes of 22 multiresistant serotype 23F isolates of S. pneumoniae from the United States and 1 isolate each from Spain and South Africa. Also included were seven multiresistant isolates of other serotypes, three penicillin-resistant but chloramphenicol-susceptible serotype 23F isolates, and two penicillin-susceptible isolates (one penicillin-susceptible isolate was serotype 23F). Fifteen of the 22 multiresistant isolates from the United States and the isolates from Spain and South Africa had identical PBP patterns, MLEE profiles, and ribotypes. Six of the remaining seven multiresistant isolates were related by PBP pattern, but demonstrated slightly different MLEE and/or ribotype profiles, possibly because of acquisition of additional resistance markers (four of the six isolates were also resistant to erythromycin). The remaining multiresistant serotype 23F isolate had a unique PBP pattern and ribotype and was only distantly related to the other pneumococcal isolates by MLEE analysis. The PBP patterns, MLEE profiles, and ribotypes of the multiresistant serotype 23F isolates were easily distinguished from those of six multiresistant isolates of other serotypes; three other penicillin-resistant, chloramphenicol-susceptible, serotype 23F isolates; and two penicillin-susceptible isolates. One exception was a multiresistant serotype 19A isolate that was highly related to the clonal group by PBP pattern and MLEE analysis and that had a ribotype similar to those of the other erythromycin-resistant serotype 23F isolates. MLEE analysis and ribotyping were more discriminating than were the PBP patterns in discerning strain differences. These data strongly suggest that a multiresistant clone of S. pneumoniae serotype 23F that is related to multiresistant isolates from Spain and South Africa has become disseminated in the United States. Clinicians should be alerted to the spread of these multiresistant strains in the United States.
Full text
PDF






Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Appelbaum P. C. World-wide development of antibiotic resistance in pneumococci. Eur J Clin Microbiol. 1987 Aug;6(4):367–377. doi: 10.1007/BF02013089. [DOI] [PubMed] [Google Scholar]
- Bonner W. M., Laskey R. A. A film detection method for tritium-labelled proteins and nucleic acids in polyacrylamide gels. Eur J Biochem. 1974 Jul 1;46(1):83–88. doi: 10.1111/j.1432-1033.1974.tb03599.x. [DOI] [PubMed] [Google Scholar]
- Broome C. V., Facklam R. R. Epidemiology of clinically significant isolates of Streptococcus pneumoniae in the United States. Rev Infect Dis. 1981 Mar-Apr;3(2):277–281. doi: 10.1093/clinids/3.2.277. [DOI] [PubMed] [Google Scholar]
- Casal J. Antimicrobial susceptibility of Streptococcus pneumoniae: serotype distribution of penicillin-resistant strains in Spain. Antimicrob Agents Chemother. 1982 Aug;22(2):222–225. doi: 10.1128/aac.22.2.222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dowson C. G., Hutchison A., Brannigan J. A., George R. C., Hansman D., Liñares J., Tomasz A., Smith J. M., Spratt B. G. Horizontal transfer of penicillin-binding protein genes in penicillin-resistant clinical isolates of Streptococcus pneumoniae. Proc Natl Acad Sci U S A. 1989 Nov;86(22):8842–8846. doi: 10.1073/pnas.86.22.8842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fenoll A., Martín Bourgon C., Muñz R., Vicioso D., Casal J. Serotype distribution and antimicrobial resistance of Streptococcus pneumoniae isolates causing systemic infections in Spain, 1979-1989. Rev Infect Dis. 1991 Jan-Feb;13(1):56–60. doi: 10.1093/clinids/13.1.56. [DOI] [PubMed] [Google Scholar]
- Geslin P., Buu-Hoi A., Frémaux A., Acar J. F. Antimicrobial resistance in Streptococcus pneumoniae: an epidemiological survey in France, 1970-1990. Clin Infect Dis. 1992 Jul;15(1):95–98. doi: 10.1093/clinids/15.1.95. [DOI] [PubMed] [Google Scholar]
- Gould F. K., Magee J. G., Ingham H. R. A hospital outbreak of antibiotic-resistant Streptococcus pneumoniae. J Infect. 1987 Jul;15(1):77–79. doi: 10.1016/s0163-4453(87)91576-3. [DOI] [PubMed] [Google Scholar]
- Gray B. M., Converse G. M., 3rd, Dillon H. C., Jr Epidemiologic studies of Streptococcus pneumoniae in infants: acquisition, carriage, and infection during the first 24 months of life. J Infect Dis. 1980 Dec;142(6):923–933. doi: 10.1093/infdis/142.6.923. [DOI] [PubMed] [Google Scholar]
- Grimont F., Grimont P. A. Ribosomal ribonucleic acid gene restriction patterns as potential taxonomic tools. Ann Inst Pasteur Microbiol. 1986 Sep-Oct;137B(2):165–175. doi: 10.1016/s0769-2609(86)80105-3. [DOI] [PubMed] [Google Scholar]
- Hakenbeck R., Briese T., Ellerbrok H. Antibodies against the benzylpenicilloyl moiety as a probe for penicillin-binding proteins. Eur J Biochem. 1986 May 15;157(1):101–106. doi: 10.1111/j.1432-1033.1986.tb09644.x. [DOI] [PubMed] [Google Scholar]
- Henderson F. W., Gilligan P. H., Wait K., Goff D. A. Nasopharyngeal carriage of antibiotic-resistant pneumococci by children in group day care. J Infect Dis. 1988 Feb;157(2):256–263. doi: 10.1093/infdis/157.2.256. [DOI] [PubMed] [Google Scholar]
- Jabes D., Nachman S., Tomasz A. Penicillin-binding protein families: evidence for the clonal nature of penicillin resistance in clinical isolates of pneumococci. J Infect Dis. 1989 Jan;159(1):16–25. doi: 10.1093/infdis/159.1.16. [DOI] [PubMed] [Google Scholar]
- Jacobs M. R., Koornhof H. J., Robins-Browne R. M., Stevenson C. M., Vermaak Z. A., Freiman I., Miller G. B., Witcomb M. A., Isaäcson M., Ward J. I. Emergence of multiply resistant pneumococci. N Engl J Med. 1978 Oct 5;299(14):735–740. doi: 10.1056/NEJM197810052991402. [DOI] [PubMed] [Google Scholar]
- Jorgensen J. H., Doern G. V., Maher L. A., Howell A. W., Redding J. S. Antimicrobial resistance among respiratory isolates of Haemophilus influenzae, Moraxella catarrhalis, and Streptococcus pneumoniae in the United States. Antimicrob Agents Chemother. 1990 Nov;34(11):2075–2080. doi: 10.1128/aac.34.11.2075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klein J. O. The epidemiology of pneumococcal disease in infants and children. Rev Infect Dis. 1981 Mar-Apr;3(2):246–253. doi: 10.1093/clinids/3.2.246. [DOI] [PubMed] [Google Scholar]
- Klugman K. P., Koornhof H. J. Drug resistance patterns and serogroups or serotypes of pneumococcal isolates from cerebrospinal fluid or blood, 1979-1986. J Infect Dis. 1988 Nov;158(5):956–964. doi: 10.1093/infdis/158.5.956. [DOI] [PubMed] [Google Scholar]
- Klugman K. P., Koornhof H. J., Kuhnle V. Clinical and nasopharyngeal isolates of unusual multiply resistant pneumococci. Am J Dis Child. 1986 Nov;140(11):1186–1190. doi: 10.1001/archpedi.1986.02140250112045. [DOI] [PubMed] [Google Scholar]
- Klugman K. P. Pneumococcal resistance to antibiotics. Clin Microbiol Rev. 1990 Apr;3(2):171–196. doi: 10.1128/cmr.3.2.171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LACKS S., HOTCHKISS R. D. A study of the genetic material determining an enzyme in Pneumococcus. Biochim Biophys Acta. 1960 Apr 22;39:508–518. doi: 10.1016/0006-3002(60)90205-5. [DOI] [PubMed] [Google Scholar]
- Latorre C., Juncosa T., Sanfeliu I. Antibiotic resistance and serotypes of 100 Streptococcus pneumoniae strains isolated in a children's hospital in Barcelona, Spain. Antimicrob Agents Chemother. 1985 Aug;28(2):357–359. doi: 10.1128/aac.28.2.357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marton A., Gulyas M., Munoz R., Tomasz A. Extremely high incidence of antibiotic resistance in clinical isolates of Streptococcus pneumoniae in Hungary. J Infect Dis. 1991 Mar;163(3):542–548. doi: 10.1093/infdis/163.3.542. [DOI] [PubMed] [Google Scholar]
- Meers P. D., Matthews R. B. Multiply resistant pneumococcus. Lancet. 1978 Jul 22;2(8082):219–219. doi: 10.1016/s0140-6736(78)91966-9. [DOI] [PubMed] [Google Scholar]
- Michel J., Dickman D., Greenberg Z., Bergner-Rabinowitz S. Serotype distribution of penicillin-resistant pneumococci and their susceptibilities to seven antimicrobial agents. Antimicrob Agents Chemother. 1983 Mar;23(3):397–401. doi: 10.1128/aac.23.3.397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moreillon P., Tomasz A. Penicillin resistance and defective lysis in clinical isolates of pneumococci: evidence for two kinds of antibiotic pressure operating in the clinical environment. J Infect Dis. 1988 Jun;157(6):1150–1157. doi: 10.1093/infdis/157.6.1150. [DOI] [PubMed] [Google Scholar]
- Mushtaque A., Chowdhury R., Vaughan J. P. Perception of diarrhoea and the use of a homemade oral rehydration solution in rural Bangladesh. J Diarrhoeal Dis Res. 1988 Mar;6(1):6–14. [PubMed] [Google Scholar]
- Muñoz R., Coffey T. J., Daniels M., Dowson C. G., Laible G., Casal J., Hakenbeck R., Jacobs M., Musser J. M., Spratt B. G. Intercontinental spread of a multiresistant clone of serotype 23F Streptococcus pneumoniae. J Infect Dis. 1991 Aug;164(2):302–306. doi: 10.1093/infdis/164.2.302. [DOI] [PubMed] [Google Scholar]
- Porter R. D., Guild W. R. Characterization of some pneumococcal bacteriophages. J Virol. 1976 Aug;19(2):659–667. doi: 10.1128/jvi.19.2.659-667.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Selander R. K., Caugant D. A., Ochman H., Musser J. M., Gilmour M. N., Whittam T. S. Methods of multilocus enzyme electrophoresis for bacterial population genetics and systematics. Appl Environ Microbiol. 1986 May;51(5):873–884. doi: 10.1128/aem.51.5.873-884.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith C. L., Cantor C. R. Purification, specific fragmentation, and separation of large DNA molecules. Methods Enzymol. 1987;155:449–467. doi: 10.1016/0076-6879(87)55030-3. [DOI] [PubMed] [Google Scholar]
- Viering T. P., Fine D. P. Genetic analysis of Streptococcus pneumoniae serotypes with the use of DNA fingerprinting. J Infect Dis. 1989 Jul;160(1):76–82. doi: 10.1093/infdis/160.1.76. [DOI] [PubMed] [Google Scholar]
- Ward J. Antibiotic-resistant Streptococcus pneumoniae: clinical and epidemiologic aspects. Rev Infect Dis. 1981 Mar-Apr;3(2):254–266. doi: 10.1093/clinids/3.2.254. [DOI] [PubMed] [Google Scholar]
- Williams W. W., Hickson M. A., Kane M. A., Kendal A. P., Spika J. S., Hinman A. R. Immunization policies and vaccine coverage among adults. The risk for missed opportunities. Ann Intern Med. 1988 Apr;108(4):616–625. doi: 10.7326/0003-4819-108-4-616. [DOI] [PubMed] [Google Scholar]
- Williamson R., Hakenbeck R., Tomasz A. In vivo interaction of beta-lactam antibiotics with the penicillin-binding proteins of Streptococcus pneumoniae. Antimicrob Agents Chemother. 1980 Oct;18(4):629–637. doi: 10.1128/aac.18.4.629. [DOI] [PMC free article] [PubMed] [Google Scholar]






