Abstract
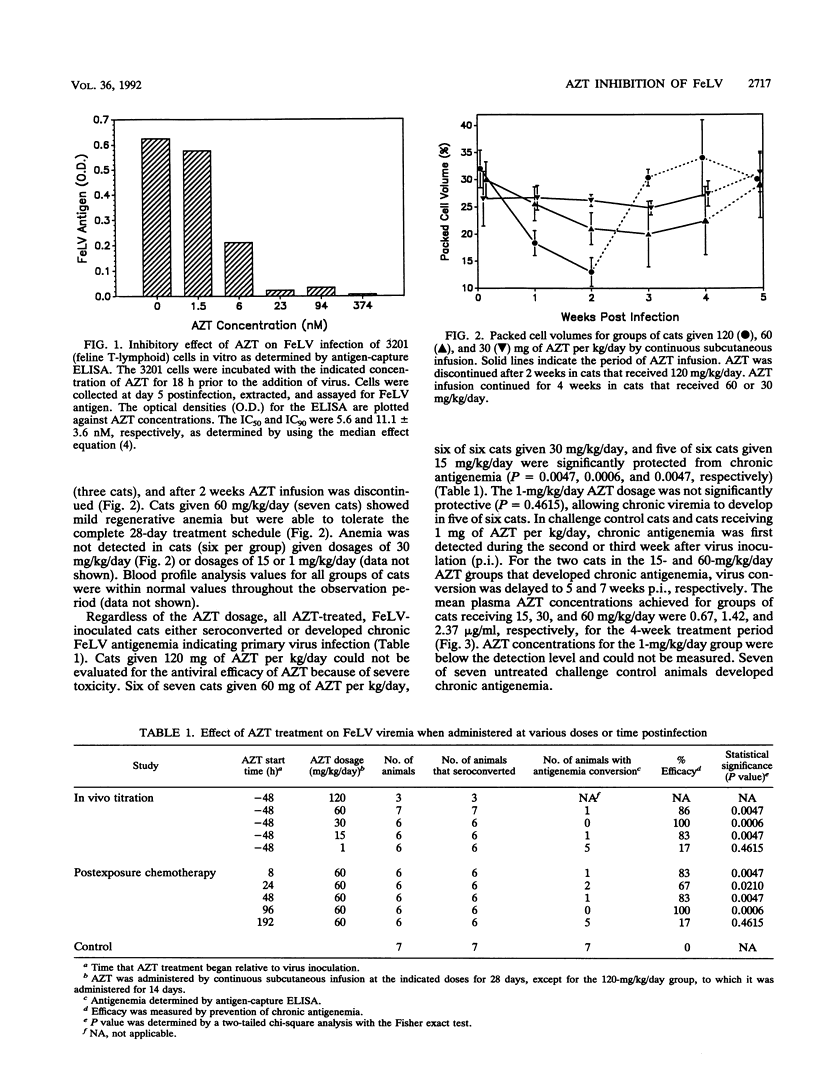
The benefits of postexposure 3'-azido-3'-dideoxythymidine (AZT) prophylaxis following human immunodeficiency virus exposure are unknown. We describe a comprehensive assessment of pre- and postexposure AZT therapy in the feline leukemia virus (FeLV)-cat model for AIDS which included in vitro testing, an in vivo dose-response titration, a postexposure treatment study, plasma drug concentration determinations, and evaluation of the immune response to FeLV. In in vitro studies, AZT prevented FeLV infection of a feline T-lymphoid cell line, giving 50 and 90% inhibition concentrations of 4.6 and 11.1 mM, respectively. In all of the in vivo efficacy studies, AZT was administered by continuous subcutaneous infusion for 28 days. AZT toxicity was excessive at a dosage of 120 mg/kg of body weight per day, causing acute anemia, but AZT was tolerable at 60 mg/kg/day. In preexposure studies, AZT was efficacious in preventing chronic antigenemia at a dosage of > or = 15 mg/kg/day, at which plasma AZT concentrations averaged between 0.51 and 0.81 micrograms/ml (2.13 and 3.03 microM). As a postexposure treatment, at 60 mg/kg/day, AZT prevented chronic FeLV antigenemia when treatment was started up to 96 h post-virus inoculation (p.i.), but not when treatment was started at 192 h p.i. The 4-day period between 96 and 192 h p.i. appears to be critical for establishing chronic viremia. It is presumed that the increase in virus load between 4 and 8 days p.i. was able to overwhelm the immunologic functions responsible for containment of FeLV infection, even though AZT therapy effectively controlled viremia during the treatment period. The antibody response to FeLV varied depending on the time of AZT treatment initiation relative to virus challenge.When AZT treatment was started 48 h before or 8 h after FeLV challenge, antibodies to FeLV were not detected until after AZT treatment was discontinued at 28 days p.i. Following AZT treatment, however, antibody titers rapidly increased at a rate suggestive of a secondary immune response. When AZT treatment was initiate at later time points relative to virus challenge (24, 48, and 96 h p.i.), antibodies to FeLV became detectable during the treatment period. These results indicate that AZT treatment does not completely prevent FeLV infection, even when treatment begins before virus challenge, and that immune sensitization to FeLV proceeds during the prophylactic drug treatment period.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Basham T., Rios C. D., Holdener T., Merigan T. C. Zidovudine (AZT) reduces virus titer, retards immune dysfunction, and prolongs survival in the LP-BM5 murine induced immunodeficiency model. J Infect Dis. 1990 May;161(5):1006–1009. doi: 10.1093/infdis/161.5.1006. [DOI] [PubMed] [Google Scholar]
- Chou T. C., Talalay P. Quantitative analysis of dose-effect relationships: the combined effects of multiple drugs or enzyme inhibitors. Adv Enzyme Regul. 1984;22:27–55. doi: 10.1016/0065-2571(84)90007-4. [DOI] [PubMed] [Google Scholar]
- Cockerell G. L., Hoover E. A., Krakowka S., Olsen R. G., Yohn D. S. Lymphocyte mitogen reactivity and enumeration of circulating B- and T-cells during feline leukemia virus infection in the cat. J Natl Cancer Inst. 1976 Nov;57(5):1095–1099. doi: 10.1093/jnci/57.5.1095. [DOI] [PubMed] [Google Scholar]
- Daar E. S., Moudgil T., Meyer R. D., Ho D. D. Transient high levels of viremia in patients with primary human immunodeficiency virus type 1 infection. N Engl J Med. 1991 Apr 4;324(14):961–964. doi: 10.1056/NEJM199104043241405. [DOI] [PubMed] [Google Scholar]
- Durand E., Le Jeunne C., Hugues F. C. Failure of prophylactic zidovudine after suicidal self-inoculation of HIV-infected blood. N Engl J Med. 1991 Apr 11;324(15):1062–1062. doi: 10.1056/NEJM199104113241513. [DOI] [PubMed] [Google Scholar]
- Essex M., Klein G., Snyder S. P., Harrold J. B. Correlation between humoral antibody and regression of tumours induced by feline sarcoma virus. Nature. 1971 Sep 17;233(5316):195–196. doi: 10.1038/233195a0. [DOI] [PubMed] [Google Scholar]
- Fischinger P. J., Blevins C. S., Nomura S. Simple, quantitative assay for both xenotropic murine leukemia and ecotropic feline leukemia viruses. J Virol. 1974 Jul;14(1):177–179. doi: 10.1128/jvi.14.1.177-179.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fischl M. A., Richman D. D., Grieco M. H., Gottlieb M. S., Volberding P. A., Laskin O. L., Leedom J. M., Groopman J. E., Mildvan D., Schooley R. T. The efficacy of azidothymidine (AZT) in the treatment of patients with AIDS and AIDS-related complex. A double-blind, placebo-controlled trial. N Engl J Med. 1987 Jul 23;317(4):185–191. doi: 10.1056/NEJM198707233170401. [DOI] [PubMed] [Google Scholar]
- Hardy W. D., Jr, Hess P. W., MacEwen E. G., McClelland A. J., Zuckerman E. E., Essex M., Cotter S. M., Jarrett O. Biology of feline leukemia virus in the natural environment. Cancer Res. 1976 Feb;36(2 Pt 2):582–588. [PubMed] [Google Scholar]
- Hom R. C., Finberg R. W., Mullaney S., Ruprecht R. M. Protective cellular retroviral immunity requires both CD4+ and CD8+ immune T cells. J Virol. 1991 Jan;65(1):220–224. doi: 10.1128/jvi.65.1.220-224.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoover E. A., Schaller J. P., Mathes L. E., Olsen R. G. Passive immunity to feline leukemia: evaluation of immunity from dams naturally infected and experimentally vaccinated. Infect Immun. 1977 Apr;16(1):54–59. doi: 10.1128/iai.16.1.54-59.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lange J. M., Boucher C. A., Hollak C. E., Wiltink E. H., Reiss P., van Royen E. A., Roos M., Danner S. A., Goudsmit J. Failure of zidovudine prophylaxis after accidental exposure to HIV-1. N Engl J Med. 1990 May 10;322(19):1375–1377. doi: 10.1056/NEJM199005103221907. [DOI] [PubMed] [Google Scholar]
- Looke D. F., Grove D. I. Failed prophylactic zidovudine after needlestick injury. Lancet. 1990 May 26;335(8700):1280–1280. doi: 10.1016/0140-6736(90)91343-9. [DOI] [PubMed] [Google Scholar]
- Lundgren B., Böttiger D., Ljungdahl-Ståhle E., Norrby E., Ståhle L., Wahren B., Oberg B. Antiviral effects of 3'-fluorothymidine and 3'-azidothymidine in cynomolgus monkeys infected with simian immunodeficiency virus. J Acquir Immune Defic Syndr. 1991;4(5):489–498. [PubMed] [Google Scholar]
- McCune J. M., Namikawa R., Shih C. C., Rabin L., Kaneshima H. Suppression of HIV infection in AZT-treated SCID-hu mice. Science. 1990 Feb 2;247(4942):564–566. doi: 10.1126/science.2300816. [DOI] [PubMed] [Google Scholar]
- Mitsuya H., Weinhold K. J., Furman P. A., St Clair M. H., Lehrman S. N., Gallo R. C., Bolognesi D., Barry D. W., Broder S. 3'-Azido-3'-deoxythymidine (BW A509U): an antiviral agent that inhibits the infectivity and cytopathic effect of human T-lymphotropic virus type III/lymphadenopathy-associated virus in vitro. Proc Natl Acad Sci U S A. 1985 Oct;82(20):7096–7100. doi: 10.1073/pnas.82.20.7096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morrey J. D., Okleberry K. M., Sidwell R. W. Early-initiated zidovudine therapy prevents disease but not low levels of persistent retrovirus in mice. J Acquir Immune Defic Syndr. 1991;4(5):506–512. [PubMed] [Google Scholar]
- Perryman L. E., Hoover E. A., Yohn D. S. Immunologic reactivity of the cat: immunosuppression in experimental feline leukemia. J Natl Cancer Inst. 1972 Nov;49(5):1357–1365. [PubMed] [Google Scholar]
- Polas P. J., Swenson C. L., Sams R., Cheney C. M., Hayes K. A., Tarr M. J., Kociba G. J., Mathes L. E. In vitro and in vivo evidence that the antiviral activity of 2',3'-dideoxycytidine is target cell dependent in a feline retrovirus animal model. Antimicrob Agents Chemother. 1990 Jul;34(7):1414–1421. doi: 10.1128/aac.34.7.1414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Portnoi D., Stall A. M., Schwartz D., Merigan T. C., Herzenberg L. A., Basham T. Zidovudine (azido dideoxythymidine) inhibits characteristic early alterations of lymphoid cell populations in retrovirus-induced murine AIDS. J Immunol. 1990 Mar 1;144(5):1705–1710. [PubMed] [Google Scholar]
- Richman D. D., Fischl M. A., Grieco M. H., Gottlieb M. S., Volberding P. A., Laskin O. L., Leedom J. M., Groopman J. E., Mildvan D., Hirsch M. S. The toxicity of azidothymidine (AZT) in the treatment of patients with AIDS and AIDS-related complex. A double-blind, placebo-controlled trial. N Engl J Med. 1987 Jul 23;317(4):192–197. doi: 10.1056/NEJM198707233170402. [DOI] [PubMed] [Google Scholar]
- Ruprecht R. M., Mullaney S., Bernard L. D., Gama Sosa M. A., Hom R. C., Finberg R. W. Vaccination with a live retrovirus: the nature of the protective immune response. Proc Natl Acad Sci U S A. 1990 Jul;87(14):5558–5562. doi: 10.1073/pnas.87.14.5558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruprecht R. M., O'Brien L. G., Rossoni L. D., Nusinoff-Lehrman S. Suppression of mouse viraemia and retroviral disease by 3'-azido-3'-deoxythymidine. Nature. 1986 Oct 2;323(6087):467–469. doi: 10.1038/323467a0. [DOI] [PubMed] [Google Scholar]
- St Clair M. H., Richards C. A., Spector T., Weinhold K. J., Miller W. H., Langlois A. J., Furman P. A. 3'-Azido-3'-deoxythymidine triphosphate as an inhibitor and substrate of purified human immunodeficiency virus reverse transcriptase. Antimicrob Agents Chemother. 1987 Dec;31(12):1972–1977. doi: 10.1128/aac.31.12.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swenson C. L., Polas P. J., Mathes L. E. A technique for continuous intravenous infusion in cats. Lab Anim Sci. 1989 Nov;39(6):615–617. [PubMed] [Google Scholar]
- Tavares L., Roneker C., Johnston K., Lehrman S. N., de Noronha F. 3'-Azido-3'-deoxythymidine in feline leukemia virus-infected cats: a model for therapy and prophylaxis of AIDS. Cancer Res. 1987 Jun 15;47(12):3190–3194. [PubMed] [Google Scholar]
- Theilen G. H., Kawakami T. G., Rush J. D., Munn R. J. Replication of cat leukemia virus in cell suspension cultures. Nature. 1969 May 10;222(5193):589–590. doi: 10.1038/222589b0. [DOI] [PubMed] [Google Scholar]
- Tremblay M., Numazaki K., Li X. G., Gornitsky M., Hiscott J., Wainberg M. A. Resistance to infection by HIV-1 of peripheral blood mononuclear cells from HIV-1-infected patients is probably mediated by neutralizing antibodies. J Immunol. 1990 Nov 1;145(9):2896–2901. [PubMed] [Google Scholar]
- Yarchoan R., Mitsuya H., Myers C. E., Broder S. Clinical pharmacology of 3'-azido-2',3'-dideoxythymidine (zidovudine) and related dideoxynucleosides. N Engl J Med. 1989 Sep 14;321(11):726–738. doi: 10.1056/NEJM198909143211106. [DOI] [PubMed] [Google Scholar]
- de Noronha F., Schäfer W., Essex M., Bolognesi D. P. Influence of antisera to oncornavirus glycoprotein (gp71) on infections of cats with feline leukemia virus. Virology. 1978 Apr;85(2):617–621. doi: 10.1016/0042-6822(78)90467-1. [DOI] [PubMed] [Google Scholar]


