Abstract
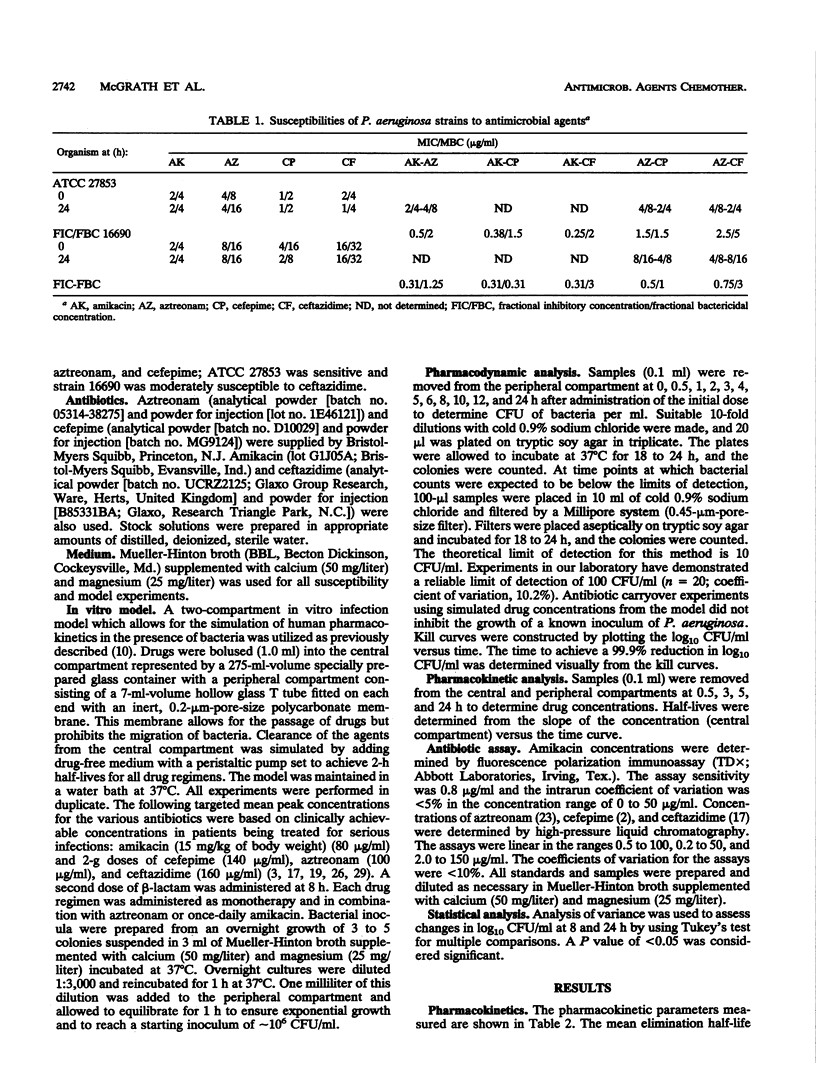
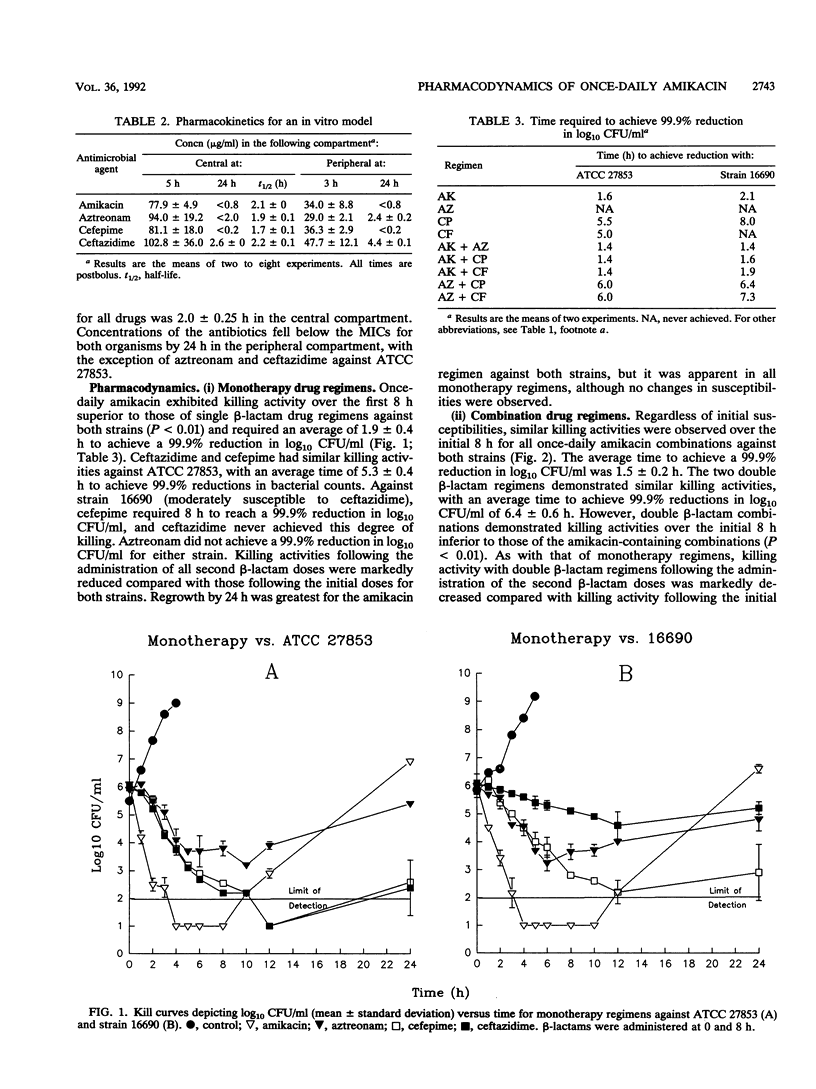
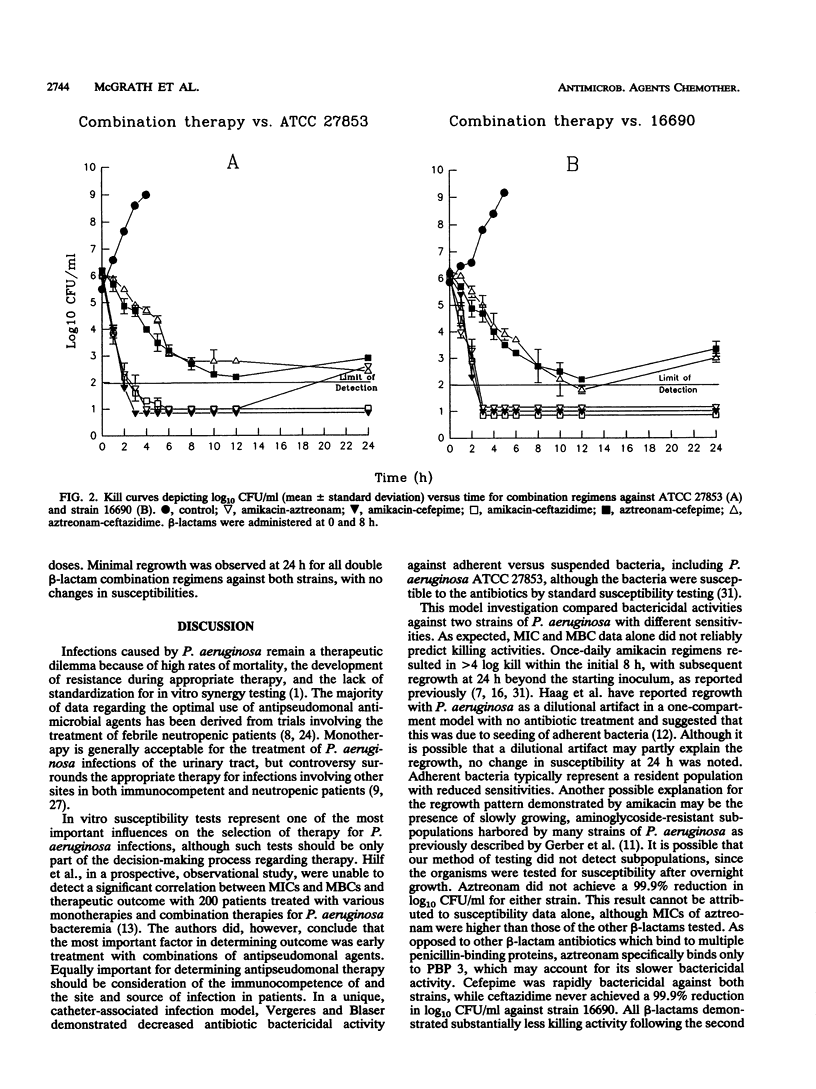
The pharmacodynamics of once-daily amikacin administered as monotherapy and in combination with aztreonam, ceftazidime, and cefepime against Pseudomonas aeruginosa ATCC 27853 and clinical isolate 16690 (moderately susceptible to ceftazidime) were investigated with an in vitro model of infection over a 24-h period. Monotherapy with aztreonam, ceftazidime, and cefepime and combinations of aztreonam with cefepime or ceftazidime were also studied. MICs and MBCs were determined for viable organisms at 24 h to test for the development of resistance. Once-daily amikacin demonstrated killing activity over the initial 8 h superior to those of all other drugs administered as monotherapy against both strains tested (P < 0.01). Regrowth by 24 h was greatest for the amikacin regimen (P < 0.01) but was apparent for all monotherapy regimens against both strains. No changes in susceptibilities were observed. All combination therapies containing once-daily amikacin achieved 99.9% reductions in log10 CFU/ml by 2.0 h against both strains, with no regrowth of organisms at 24 h. Aztreonam-cefepime and -ceftazidime combinations required approximately 5 to 6 h to achieve a 99.9% reduction in log10 CFU/ml. Although there was no difference in time to the 99.9% kill between the aztreonam-cefepime and -ceftazidime regimens against either strain, the killing activity of these combinations was significantly less than those of regimens containing once-daily amikacin (P < 0.01). Minor differences in the initial susceptibilities of beta-lactams and the monobactam aztreonam against P. aeruginosa may not be important for therapeutic outcomes when used in combination with single-daily aminoglycoside therapy.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Allan J. D., Moellering R. C., Jr Management of infections caused by gram-negative bacilli: the role of antimicrobial combinations. Rev Infect Dis. 1985 Nov-Dec;7 (Suppl 4):S559–S571. doi: 10.1093/clinids/7.supplement_4.s559. [DOI] [PubMed] [Google Scholar]
- Barbhaiya R. H., Forgue S. T., Shyu W. C., Papp E. A., Pittman K. A. High-pressure liquid chromatographic analysis of BMY-28142 in plasma and urine. Antimicrob Agents Chemother. 1987 Jan;31(1):55–59. doi: 10.1128/aac.31.1.55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barbhaiya R. H., Knupp C. A., Forgue S. T., Matzke G. R., Halstenson C. E., Opsahl J. A., Pittman K. A. Disposition of the cephalosporin cefepime in normal and renally impaired subjects. Drug Metab Dispos. 1991 Jan-Feb;19(1):68–73. [PubMed] [Google Scholar]
- Craig W. A., Vogelman B. The postantibiotic effect. Ann Intern Med. 1987 Jun;106(6):900–902. doi: 10.7326/0003-4819-106-6-900. [DOI] [PubMed] [Google Scholar]
- De Jongh C. A., Joshi J. H., Thompson B. W., Newman K. A., Finley R. S., Moody M. R., Salvatore P. C., Tenney J. H., Drusano G. L., Schimpff S. C. A double beta-lactam combination versus an aminoglycoside-containing regimen as empiric antibiotic therapy for febrile granulocytopenic cancer patients. Am J Med. 1986 May 30;80(5C):101–111. [PubMed] [Google Scholar]
- Dudley M. N., Zinner S. H. Single daily dosing of amikacin in an in-vitro model. J Antimicrob Chemother. 1991 May;27 (Suppl 100):15–19. doi: 10.1093/jac/27.suppl_c.15. [DOI] [PubMed] [Google Scholar]
- Fantin B., Carbon C. In vivo antibiotic synergism: contribution of animal models. Antimicrob Agents Chemother. 1992 May;36(5):907–912. doi: 10.1128/aac.36.5.907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gerber A. U., Vastola A. P., Brandel J., Craig W. A. Selection of aminoglycoside-resistant variants of Pseudomonas aeruginosa in an in vivo model. J Infect Dis. 1982 Nov;146(5):691–697. doi: 10.1093/infdis/146.5.691. [DOI] [PubMed] [Google Scholar]
- Hilf M., Yu V. L., Sharp J., Zuravleff J. J., Korvick J. A., Muder R. R. Antibiotic therapy for Pseudomonas aeruginosa bacteremia: outcome correlations in a prospective study of 200 patients. Am J Med. 1989 Nov;87(5):540–546. doi: 10.1016/s0002-9343(89)80611-4. [DOI] [PubMed] [Google Scholar]
- Hollender L. F., Bahnini J., De Manzini N., Lau W. Y., Fan S. T., Hermansyur K., Benny P., Husni A. N., Sutjipto, Lorber R. R. A multicentric study of netilmicin once daily versus thrice daily in patients with appendicitis and other intra-abdominal infections. J Antimicrob Chemother. 1989 May;23(5):773–783. doi: 10.1093/jac/23.5.773. [DOI] [PubMed] [Google Scholar]
- Kapusnik J. E., Hackbarth C. J., Chambers H. F., Carpenter T., Sande M. A. Single, large, daily dosing versus intermittent dosing of tobramycin for treating experimental pseudomonas pneumonia. J Infect Dis. 1988 Jul;158(1):7–12. doi: 10.1093/infdis/158.1.7. [DOI] [PubMed] [Google Scholar]
- LeBel M., Barbeau G., Vallee F., Bergeron M. G. Pharmacokinetics of ceftazidime in elderly volunteers. Antimicrob Agents Chemother. 1985 Nov;28(5):713–715. doi: 10.1128/aac.28.5.713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maller R., Isaksson B., Nilsson L., Sörén L. A study of amikacin given once versus twice daily in serious infections. J Antimicrob Chemother. 1988 Jul;22(1):75–79. doi: 10.1093/jac/22.1.75. [DOI] [PubMed] [Google Scholar]
- Mattie H. Clinical pharmacokinetics of aztreonam. Clin Pharmacokinet. 1988 Mar;14(3):148–155. doi: 10.2165/00003088-198814030-00003. [DOI] [PubMed] [Google Scholar]
- Nordström L., Ringberg H., Cronberg S., Tjernström O., Walder M. Does administration of an aminoglycoside in a single daily dose affect its efficacy and toxicity? J Antimicrob Chemother. 1990 Jan;25(1):159–173. doi: 10.1093/jac/25.1.159. [DOI] [PubMed] [Google Scholar]
- Odenholt-Tornqvist I., Löwdin E., Cars O. Pharmacodynamic effects of subinhibitory concentrations of beta-lactam antibiotics in vitro. Antimicrob Agents Chemother. 1991 Sep;35(9):1834–1839. doi: 10.1128/aac.35.9.1834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pizzo P. A., Hathorn J. W., Hiemenz J., Browne M., Commers J., Cotton D., Gress J., Longo D., Marshall D., McKnight J. A randomized trial comparing ceftazidime alone with combination antibiotic therapy in cancer patients with fever and neutropenia. N Engl J Med. 1986 Aug 28;315(9):552–558. doi: 10.1056/NEJM198608283150905. [DOI] [PubMed] [Google Scholar]
- Powell S. H., Thompson W. L., Luthe M. A., Stern R. C., Grossniklaus D. A., Bloxham D. D., Groden D. L., Jacobs M. R., DiScenna A. O., Cash H. A. Once-daily vs. continuous aminoglycoside dosing: efficacy and toxicity in animal and clinical studies of gentamicin, netilmicin, and tobramycin. J Infect Dis. 1983 May;147(5):918–932. doi: 10.1093/infdis/147.5.918. [DOI] [PubMed] [Google Scholar]
- Richards D. M., Brogden R. N. Ceftazidime. A review of its antibacterial activity, pharmacokinetic properties and therapeutic use. Drugs. 1985 Feb;29(2):105–161. doi: 10.2165/00003495-198529020-00002. [DOI] [PubMed] [Google Scholar]
- Sturm A. W. Netilmicin in the treatment of gram-negative bacteremia: single daily versus multiple daily dosage. J Infect Dis. 1989 May;159(5):931–937. doi: 10.1093/infdis/159.5.931. [DOI] [PubMed] [Google Scholar]
- Van der Auwera P., Klastersky J. Serum bactericidal activity and postantibiotic effect in serum of patients with urinary tract infection receiving high-dose amikacin. Antimicrob Agents Chemother. 1987 Jul;31(7):1061–1068. doi: 10.1128/aac.31.7.1061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vergères P., Blaser J. Amikacin, ceftazidime, and flucloxacillin against suspended and adherent Pseudomonas aeruginosa and Staphylococcus epidermidis in an in vitro model of infection. J Infect Dis. 1992 Feb;165(2):281–289. doi: 10.1093/infdis/165.2.281. [DOI] [PubMed] [Google Scholar]
- Wood C. A., Norton D. R., Kohlhepp S. J., Kohnen P. W., Porter G. A., Houghton D. C., Brummett R. E., Bennett W. M., Gilbert D. N. The influence of tobramycin dosage regimens on nephrotoxicity, ototoxicity, and antibacterial efficacy in a rat model of subcutaneous abscess. J Infect Dis. 1988 Jul;158(1):13–22. doi: 10.1093/infdis/158.1.13. [DOI] [PubMed] [Google Scholar]
- Zinner S. H., Blaser J., Stone B. B., Groner M. C. Use of an in-vitro kinetic model to study antibiotic combinations. J Antimicrob Chemother. 1985 Jan;15 (Suppl A):221–226. doi: 10.1093/jac/15.suppl_a.221. [DOI] [PubMed] [Google Scholar]


