Abstract
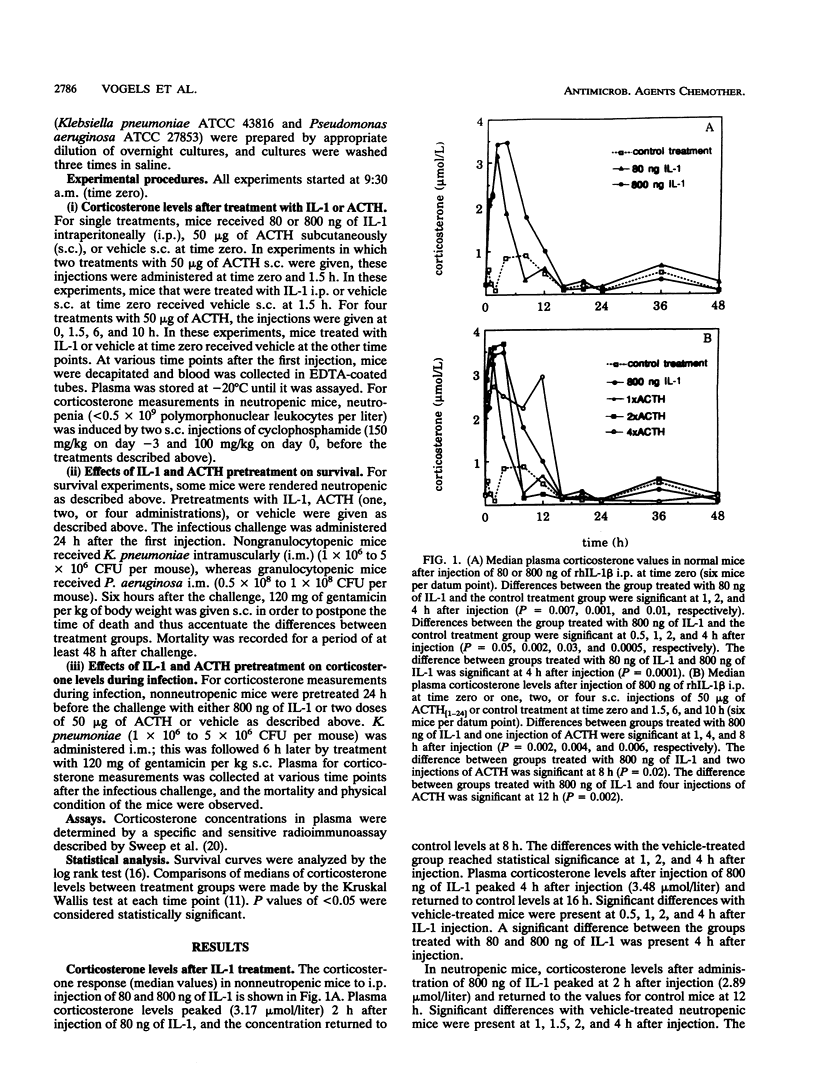
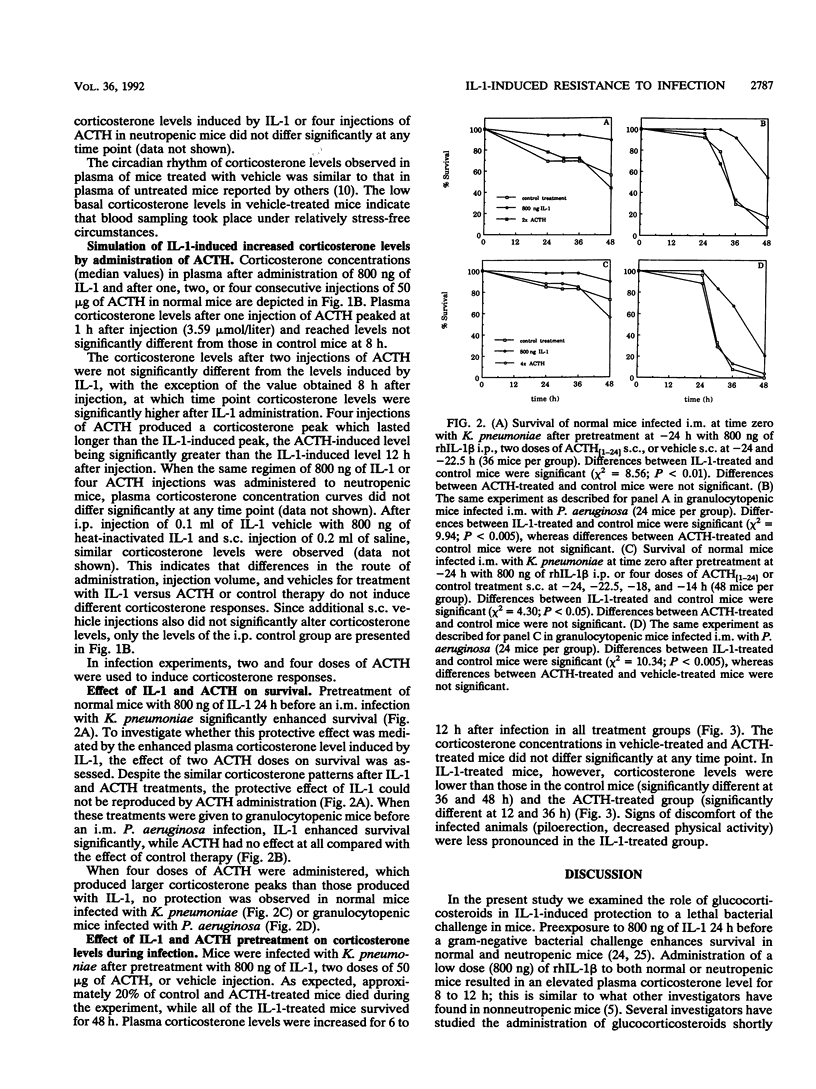
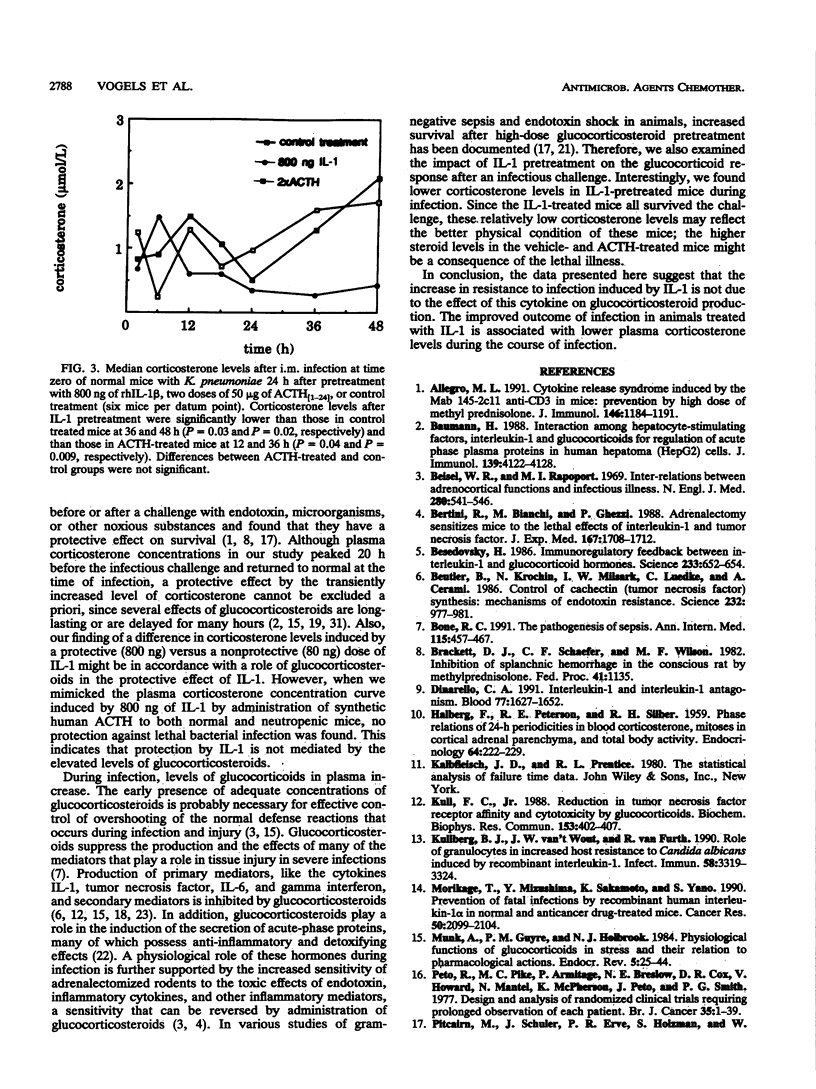
Preexposure to a low dose of interleukin-1 (IL-1; 3 to 30 micrograms/kg) 24 h before a lethal gram-negative bacterial infection prolonged survival in normal and granulocytopenic mice. To examine whether this protective effect is mediated by glucocorticosteroids, we first measured corticosterone concentrations in mice after administration of 80 and 800 ng of IL-1. IL-1 induced a dose-dependent increase in corticosterone levels in plasma. Next, the corticosterone peak induced by a protective dose of IL-1 (800 ng) was simulated by administration of synthetic human adrenocorticotropic hormone 1-24 (ACTH) in normal and neutropenic mice. Although corticosterone levels induced by pretreatment with IL-1 or ACTH were virtually identical, the ACTH-induced corticosterone peak was not associated with protection against Klebsiella pneumoniae infection in normal mice and Pseudomonas aeruginosa infection in neutropenic mice. This indicates that the protective effect of IL-1 pretreatment against gram-negative bacterial infection is not mediated by elevated levels of glucocorticosteroids. In addition, we found that plasma corticosterone concentrations during K. pneumoniae infection were significantly lower after pretreatment with IL-1 than after pretreatment with ACTH or vehicle, probably reflecting the better physical condition of IL-1-treated mice.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Alegre M. L., Vandenabeele P., Depierreux M., Florquin S., Deschodt-Lanckman M., Flamand V., Moser M., Leo O., Urbain J., Fiers W. Cytokine release syndrome induced by the 145-2C11 anti-CD3 monoclonal antibody in mice: prevention by high doses of methylprednisolone. J Immunol. 1991 Feb 15;146(4):1184–1191. [PubMed] [Google Scholar]
- Bertini R., Bianchi M., Ghezzi P. Adrenalectomy sensitizes mice to the lethal effects of interleukin 1 and tumor necrosis factor. J Exp Med. 1988 May 1;167(5):1708–1712. doi: 10.1084/jem.167.5.1708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Besedovsky H., del Rey A., Sorkin E., Dinarello C. A. Immunoregulatory feedback between interleukin-1 and glucocorticoid hormones. Science. 1986 Aug 8;233(4764):652–654. doi: 10.1126/science.3014662. [DOI] [PubMed] [Google Scholar]
- Bone R. C. The pathogenesis of sepsis. Ann Intern Med. 1991 Sep 15;115(6):457–469. doi: 10.7326/0003-4819-115-6-457. [DOI] [PubMed] [Google Scholar]
- Dinarello C. A. Interleukin-1 and interleukin-1 antagonism. Blood. 1991 Apr 15;77(8):1627–1652. [PubMed] [Google Scholar]
- Kull F. C., Jr Reduction in tumor necrosis factor receptor affinity and cytotoxicity by glucocorticoids. Biochem Biophys Res Commun. 1988 May 31;153(1):402–409. doi: 10.1016/s0006-291x(88)81238-5. [DOI] [PubMed] [Google Scholar]
- Munck A., Guyre P. M., Holbrook N. J. Physiological functions of glucocorticoids in stress and their relation to pharmacological actions. Endocr Rev. 1984 Winter;5(1):25–44. doi: 10.1210/edrv-5-1-25. [DOI] [PubMed] [Google Scholar]
- Peto R., Pike M. C., Armitage P., Breslow N. E., Cox D. R., Howard S. V., Mantel N., McPherson K., Peto J., Smith P. G. Design and analysis of randomized clinical trials requiring prolonged observation of each patient. II. analysis and examples. Br J Cancer. 1977 Jan;35(1):1–39. doi: 10.1038/bjc.1977.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pitcairn M., Schuler J., Erve P. R., Holtzman S., Schumer W. Glucocorticoid and antibiotic effect on experimental gram-negative bacteremic shock. Arch Surg. 1975 Aug;110(8):1012–1015. doi: 10.1001/archsurg.1975.01360140156030. [DOI] [PubMed] [Google Scholar]
- Rees D. D., Cellek S., Palmer R. M., Moncada S. Dexamethasone prevents the induction by endotoxin of a nitric oxide synthase and the associated effects on vascular tone: an insight into endotoxin shock. Biochem Biophys Res Commun. 1990 Dec 14;173(2):541–547. doi: 10.1016/s0006-291x(05)80068-3. [DOI] [PubMed] [Google Scholar]
- Snyers L., De Wit L., Content J. Glucocorticoid up-regulation of high-affinity interleukin 6 receptors on human epithelial cells. Proc Natl Acad Sci U S A. 1990 Apr;87(7):2838–2842. doi: 10.1073/pnas.87.7.2838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sweep C. G., van der Meer M. J., Hermus A. R., Smals A. G., van der Meer J. W., Pesman G. J., Willemsen S. J., Benraad T. J., Kloppenborg P. W. Chronic stimulation of the pituitary-adrenal axis in rats by interleukin-1 beta infusion: in vivo and in vitro studies. Endocrinology. 1992 Mar;130(3):1153–1164. doi: 10.1210/endo.130.3.1311230. [DOI] [PubMed] [Google Scholar]
- Thomas C. S., Jr, Brockman S. K. The role of adrenal corticosteroid therapy in Escherichia coli endotoxin shock. Surg Gynecol Obstet. 1968 Jan;126(1):61–69. [PubMed] [Google Scholar]
- Thompson W. L., Abeles F. B., Beall F. A., Dinterman R. E., Wannemacher R. W., Jr Influence of the adrenal glucocorticoids on the stimulation of synthesis of hepatic ribonucleic acid and plasma acute-phase globulins by leucocytic endogenous mediator. Biochem J. 1976 Apr 15;156(1):25–32. doi: 10.1042/bj1560025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vadas P., Pruzanski W. Phospholipase A2 activation is the pivotal step in the effector pathway of inflammation. Adv Exp Med Biol. 1990;275:83–101. doi: 10.1007/978-1-4684-5805-3_5. [DOI] [PubMed] [Google Scholar]
- Van der Meer J. W., Helle M., Aarden L. Comparison of the effects of recombinant interleukin 6 and recombinant interleukin 1 on nonspecific resistance to infection. Eur J Immunol. 1989 Feb;19(2):413–416. doi: 10.1002/eji.1830190229. [DOI] [PubMed] [Google Scholar]
- Van't Wout J. W., Van der Meer J. W., Barza M., Dinarello C. A. Protection of neutropenic mice from lethal Candida albicans infection by recombinant interleukin 1. Eur J Immunol. 1988 Jul;18(7):1143–1146. doi: 10.1002/eji.1830180728. [DOI] [PubMed] [Google Scholar]
- Vogels M. T., van der Meer J. W. Use of immune modulators in nonspecific therapy of bacterial infections. Antimicrob Agents Chemother. 1992 Jan;36(1):1–5. doi: 10.1128/aac.36.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Webel M. L., Ritts R. E., Jr, Taswell H. F., Danadio J. V., Jr, Woods J. E. Cellular immunity after intravenous administration of methylprednisolone. J Lab Clin Med. 1974 Mar;83(3):383–392. [PubMed] [Google Scholar]
- van der Meer J. W., Barza M., Wolff S. M., Dinarello C. A. A low dose of recombinant interleukin 1 protects granulocytopenic mice from lethal gram-negative infection. Proc Natl Acad Sci U S A. 1988 Mar;85(5):1620–1623. doi: 10.1073/pnas.85.5.1620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van der Meer J. W. The effects of recombinant interleukin-1 and recombinant tumor necrosis factor on non-specific resistance to infection. Biotherapy. 1988;1(1):19–25. doi: 10.1007/BF02170132. [DOI] [PubMed] [Google Scholar]


