Abstract
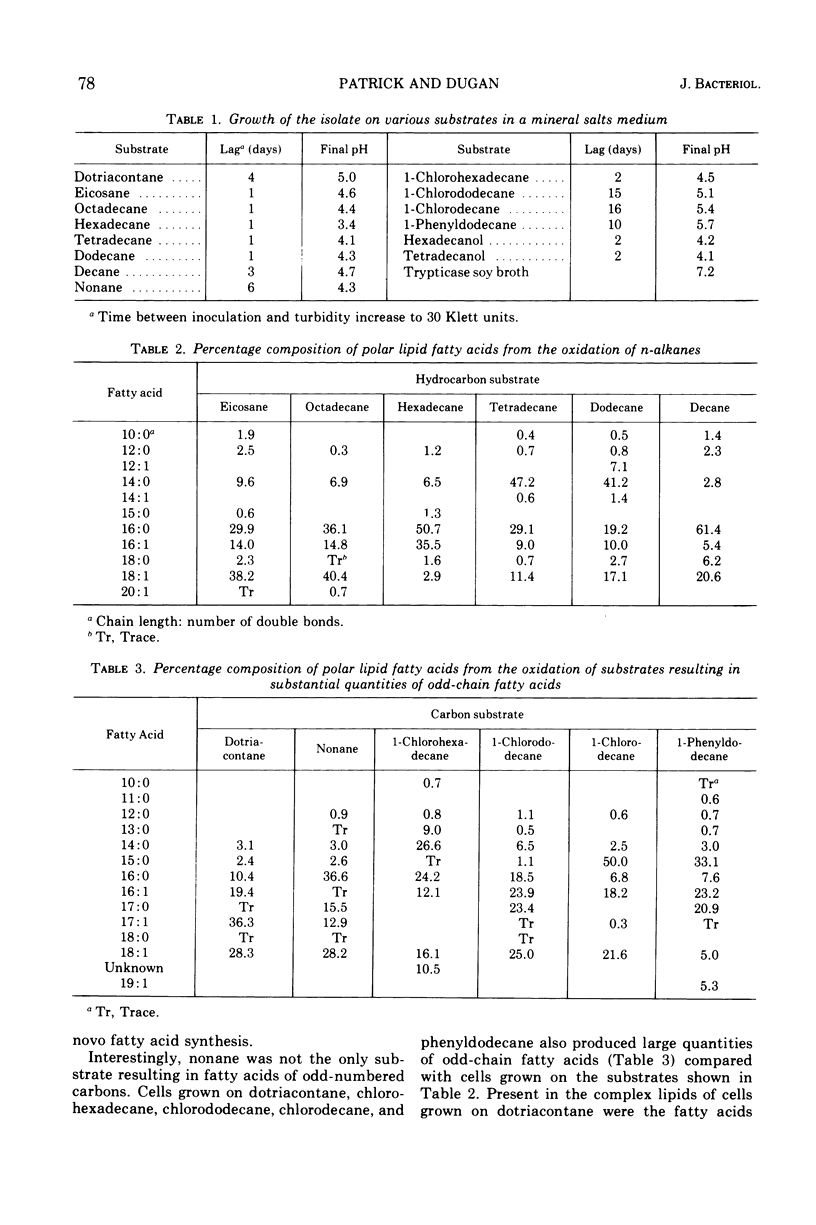
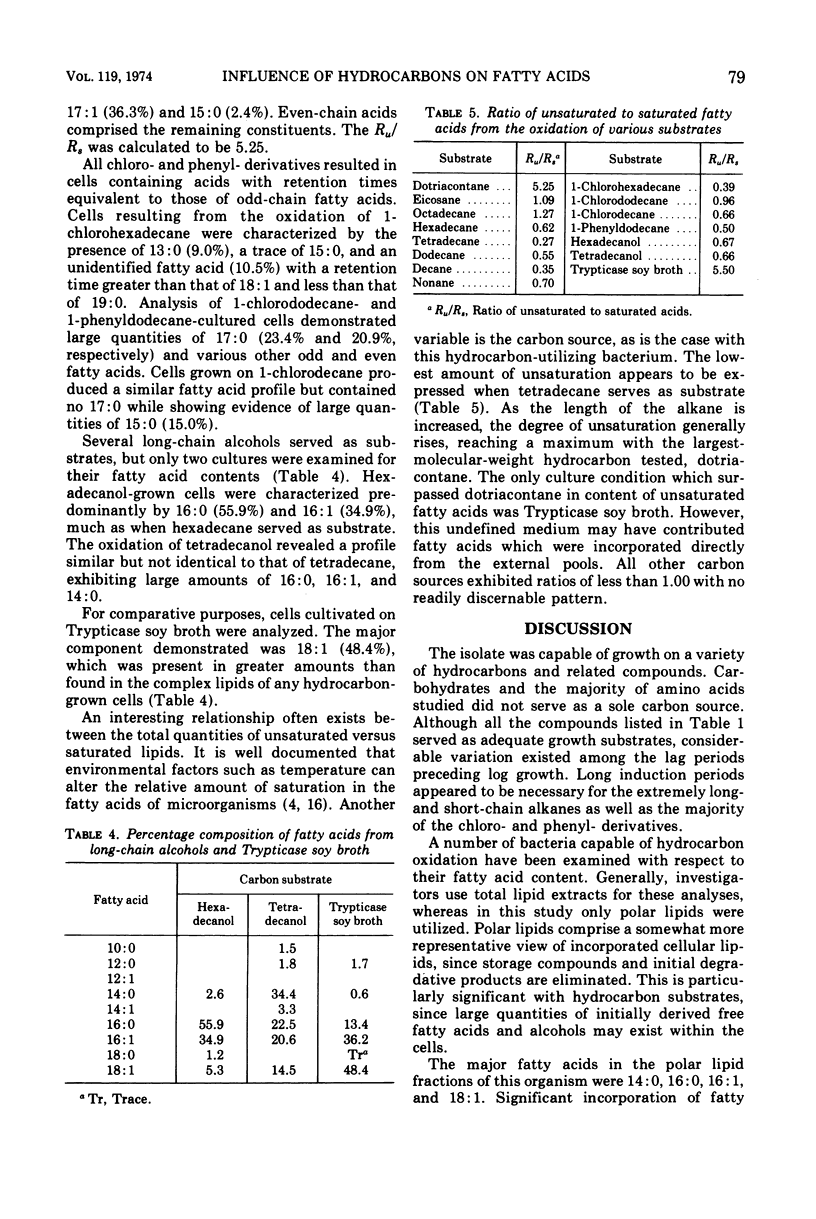
The effects of hydrocarbons and hydrocarbon derivatives as growth substrates on the polar lipid fractions of an Acinetobacter isolate were studied. Tetradecane, hexadecane, and octadecane resulted in the incorporation of substantial quantities of equivalent-chain-length fatty acids into cellular lipids. Cells cultured on nonane, the only odd-numbered alkane tested, contained both odd- and even-chain fatty acids. The n-alkane dotriacontane (32 carbons), 1-chlorohexadecane, 1-chlorododecane, 1-chlorodecane, and 1-phenyldodecane yielded significant amounts of odd-chain fatty acids. A subterminal oxidative pathway is believed to account for these results. Cells grown on long-chain alcohols exhibited fatty acid profiles nearly identical to those of cells grown on the corresponding alkanes.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Baumann P., Doudoroff M., Stanier R. Y. A study of the Moraxella group. II. Oxidative-negative species (genus Acinetobacter). J Bacteriol. 1968 May;95(5):1520–1541. doi: 10.1128/jb.95.5.1520-1541.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baumann P. Isolation of Acinetobacter from soil and water. J Bacteriol. 1968 Jul;96(1):39–42. doi: 10.1128/jb.96.1.39-42.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brian B. L., Gardner E. W. A simple procedure for detecting the presence of cyclopropane fatty acids in bacterial lipids. Appl Microbiol. 1968 Apr;16(4):549–552. doi: 10.1128/am.16.4.549-552.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DAVIS J. B. MICROBIAL INCORPORATION OF FATTY ACIDS DERIVED FROM N-ALKANES INTO GLYCERIDES AND WAXES. Appl Microbiol. 1964 May;12:210–214. doi: 10.1128/am.12.3.210-214.1964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daron H. H. Fatty acid composition of lipid extracts of a thermophilic Bacillus species. J Bacteriol. 1970 Jan;101(1):145–151. doi: 10.1128/jb.101.1.145-151.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dunlap K. R., Perry J. J. Effect of substrate on the fatty acid composition of hydrocabon-utilizing microorganisms. J Bacteriol. 1967 Dec;94(6):1919–1923. doi: 10.1128/jb.94.6.1919-1923.1967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- FOLCH J., LEES M., SLOANE STANLEY G. H. A simple method for the isolation and purification of total lipides from animal tissues. J Biol Chem. 1957 May;226(1):497–509. [PubMed] [Google Scholar]
- FUHS G. W. [Microbial catabolism of hydrocarbons]. Arch Mikrobiol. 1961;39:374–422. [PubMed] [Google Scholar]
- Forney F. W., Markovetz A. J. Subterminal oxidation of aliphatic hydrocarbons. J Bacteriol. 1970 Apr;102(1):281–282. doi: 10.1128/jb.102.1.281-282.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fredricks K. M. Products of the oxidation of n-decane by Pseudomonas aeruginosa and Mycobacterium rhodochrous. Antonie Van Leeuwenhoek. 1967;33(1):41–48. doi: 10.1007/BF02045532. [DOI] [PubMed] [Google Scholar]
- Hankin L., Kolattukudy P. E. Metabolism of a plant wax paraffin (n-nonacosane) by a soil bacterium (Micrococcus cerificans). J Gen Microbiol. 1968 May;51(3):457–463. doi: 10.1099/00221287-51-3-457. [DOI] [PubMed] [Google Scholar]
- Juni E. Interspecies transformation of Acinetobacter: genetic evidence for a ubiquitous genus. J Bacteriol. 1972 Nov;112(2):917–931. doi: 10.1128/jb.112.2.917-931.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LUKINS H. B., FOSTER J. W. METHYL KETONE METABOLISM IN HYDROCARBON-UTILIZING MYCOBACTERIA. J Bacteriol. 1963 May;85:1074–1087. doi: 10.1128/jb.85.5.1074-1087.1963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Makula R., Finnerty W. R. Microbial assimilation of hydrocarbons. I. Fatty acids derived from normal alkanes. J Bacteriol. 1968 Jun;95(6):2102–2107. doi: 10.1128/jb.95.6.2102-2107.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]


