Abstract
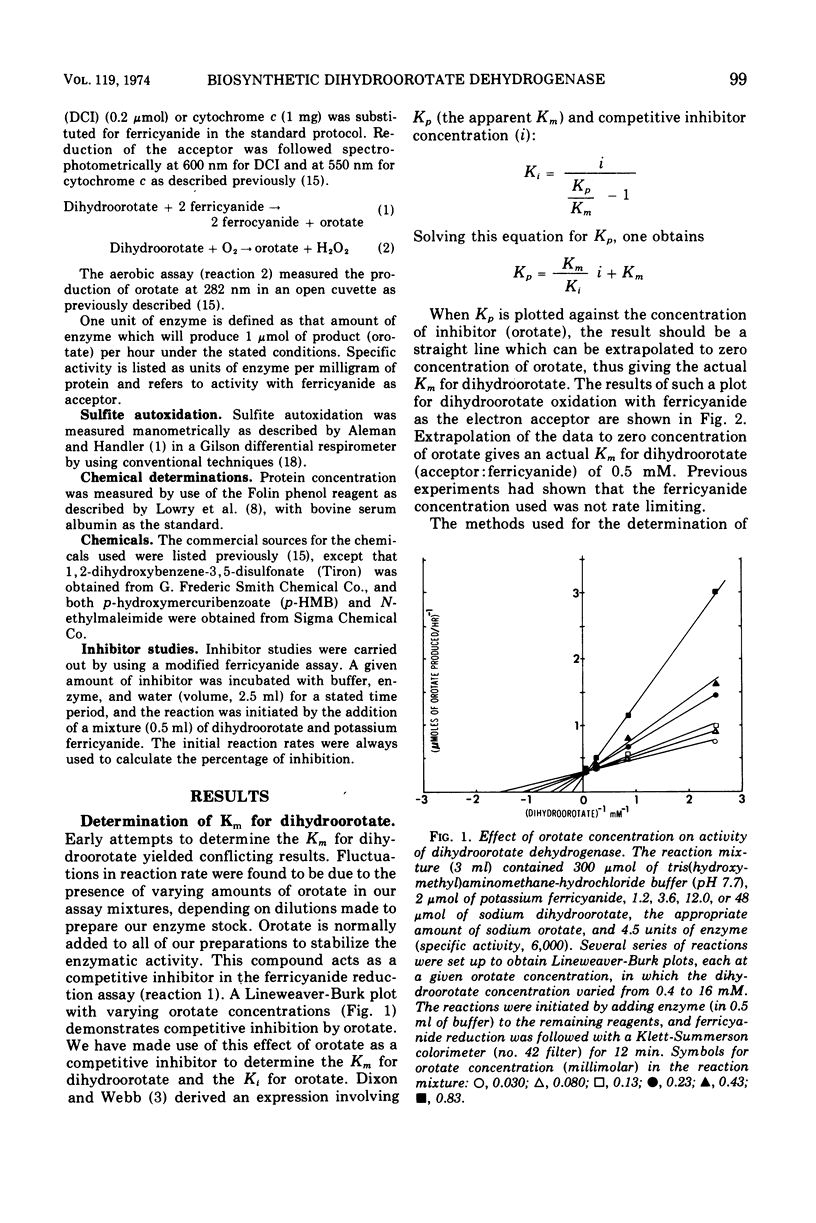
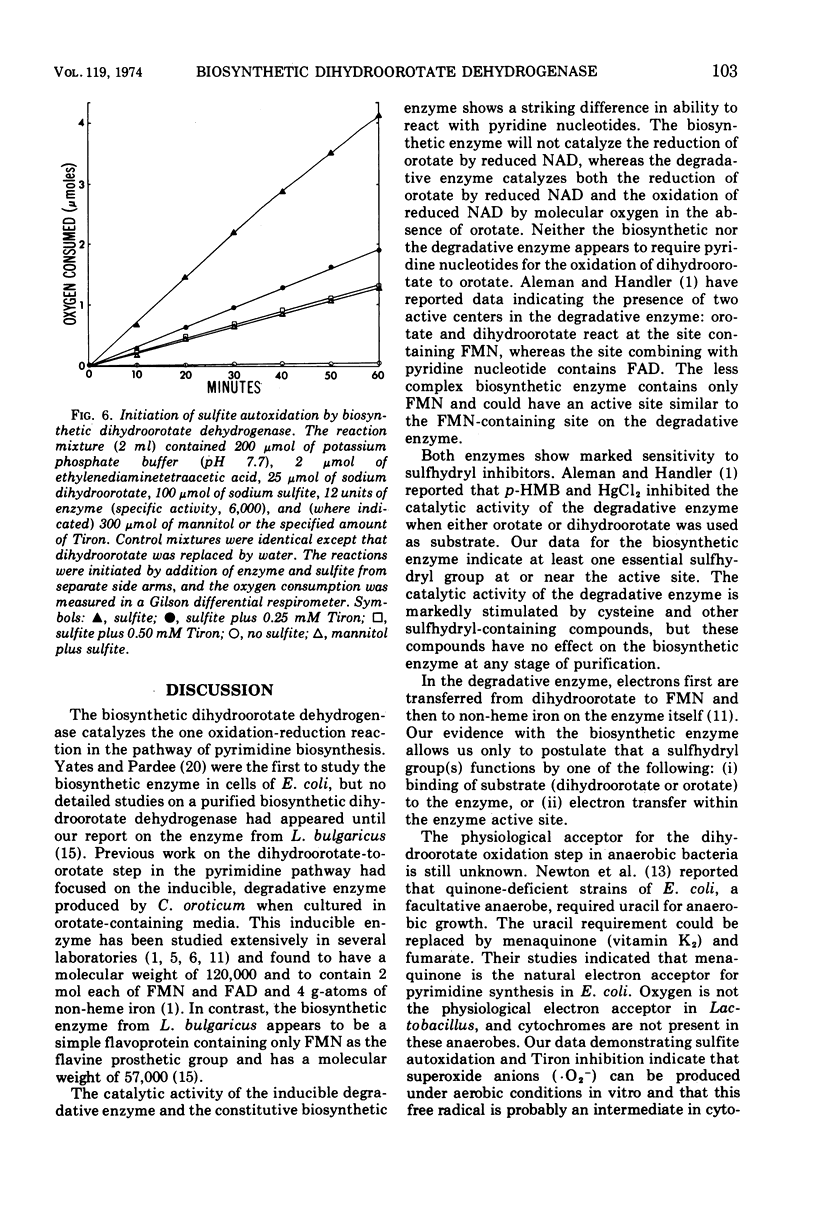
Some of the catalytic properties of the biosynthetic dihydroorotate dehydrogenase purified from an anaerobic bacterium, Lactobacillus bulgaricus, are described. Studies with p-hydroxymercuribenzoate, N-ethylmaleimide, and mercuric chloride showed that sulfhydryl groups are necessary for transfer of electrons from dihydroorotate to a variety of electron acceptors. Protection studies with substrates for the enzyme indicated that free sulfhydryl groups at or near the active center are required for catalytic activity. Evidence is presented for the production of superoxide free radicals during reaction of the enzyme with molecular oxygen. Inhibitor studies with Tiron indicated that reduction of cytochrome c by the enzyme may involve the superoxide free radical as an intermediate. Orotate, one of the substrates for the enzyme, has been found to be a competitive inhibitor for the dihydroorotate site. The Ki for orotate as estimated by several techniques is 0.1 mM. The Km for dihydroorotate with ferricyanide as the electron acceptor is estimated to be 0.5 mM.
Full text
PDF





Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aleman V., Handler P. Dihydroorotate dehydrogenase. I. General properties. J Biol Chem. 1967 Sep 25;242(18):4087–4096. [PubMed] [Google Scholar]
- FRIDOVICH I., HANDLER P. Detection of free radicals generated during enzymic oxidations by the initiation of sulfite oxidation. J Biol Chem. 1961 Jun;236:1836–1840. [PubMed] [Google Scholar]
- FRIEDMANN H. C., VENNESLAND B. Crystalline dihydroorotic dehydrogenase. J Biol Chem. 1960 May;235:1526–1532. [PubMed] [Google Scholar]
- FRIEDMANN H. C., VENNESLAND B. Purification and properties of dihydro-orotic dehydrogenase. J Biol Chem. 1958 Dec;233(6):1398–1406. [PubMed] [Google Scholar]
- Karibian D. Dihydro-orotate dehydrogenase of Escherichia coli K12: effects of triton X-100 and phospholipids. Biochim Biophys Acta. 1973 Apr 12;302(2):205–215. doi: 10.1016/0005-2744(73)90149-6. [DOI] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- MILLER R. W., MASSEY V. DIHYDROOROTIC DEHYDROGENASE. I. SOME PROPERTIES OF THE ENZYME. J Biol Chem. 1965 Mar;240:1453–1465. [PubMed] [Google Scholar]
- Massey V., Strickland S., Mayhew S. G., Howell L. G., Engel P. C., Matthews R. G., Schuman M., Sullivan P. A. The production of superoxide anion radicals in the reaction of reduced flavins and flavoproteins with molecular oxygen. Biochem Biophys Res Commun. 1969 Sep 10;36(6):891–897. doi: 10.1016/0006-291x(69)90287-3. [DOI] [PubMed] [Google Scholar]
- Miller R. W. Reactions of superoxide anion, catechols, and cytochrome c. Can J Biochem. 1970 Aug;48(8):935–939. doi: 10.1139/o70-145. [DOI] [PubMed] [Google Scholar]
- Nelson C. A., Handler P. Preparation of bovine xanthine oxidase and the subunit structures of some iron flavoproteins. J Biol Chem. 1968 Oct 25;243(20):5368–5373. [PubMed] [Google Scholar]
- Newton N. A., Cox G. B., Gibson F. The function of menaquinone (vitamin K 2 ) in Escherichia coli K-12. Biochim Biophys Acta. 1971 Jul 20;244(1):155–166. doi: 10.1016/0304-4165(71)90132-2. [DOI] [PubMed] [Google Scholar]
- Orme-Johnson W. H., Beinert H. On the formation of the superoxide anion radical during the reaction of reduced iron-sulfur proteins with oxygen. Biochem Biophys Res Commun. 1969 Sep 10;36(6):905–911. doi: 10.1016/0006-291x(69)90289-7. [DOI] [PubMed] [Google Scholar]
- PARDEE A. B., YATES R. A. Pyrimidine biosynthesis in Escherichia coli. J Biol Chem. 1956 Aug;221(2):743–756. [PubMed] [Google Scholar]
- TAYLOR W. H., TAYLOR M. L. ENZYMES OF THE PYRIMIDINE PATHWAY IN ESCHERICHIA COLI. II. INTRACELLULAR LOCALIZATION AND PROPERTIES OF DIHYDROOROTIC DEHYDROGENASE. J Bacteriol. 1964 Jul;88:105–110. doi: 10.1128/jb.88.1.105-110.1964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor W. H., Taylor M. L., Eames D. F. Two functionally different dihydroorotic dehydrogenases in bacteria. J Bacteriol. 1966 Jun;91(6):2251–2256. doi: 10.1128/jb.91.6.2251-2256.1966. [DOI] [PMC free article] [PubMed] [Google Scholar]


