Abstract
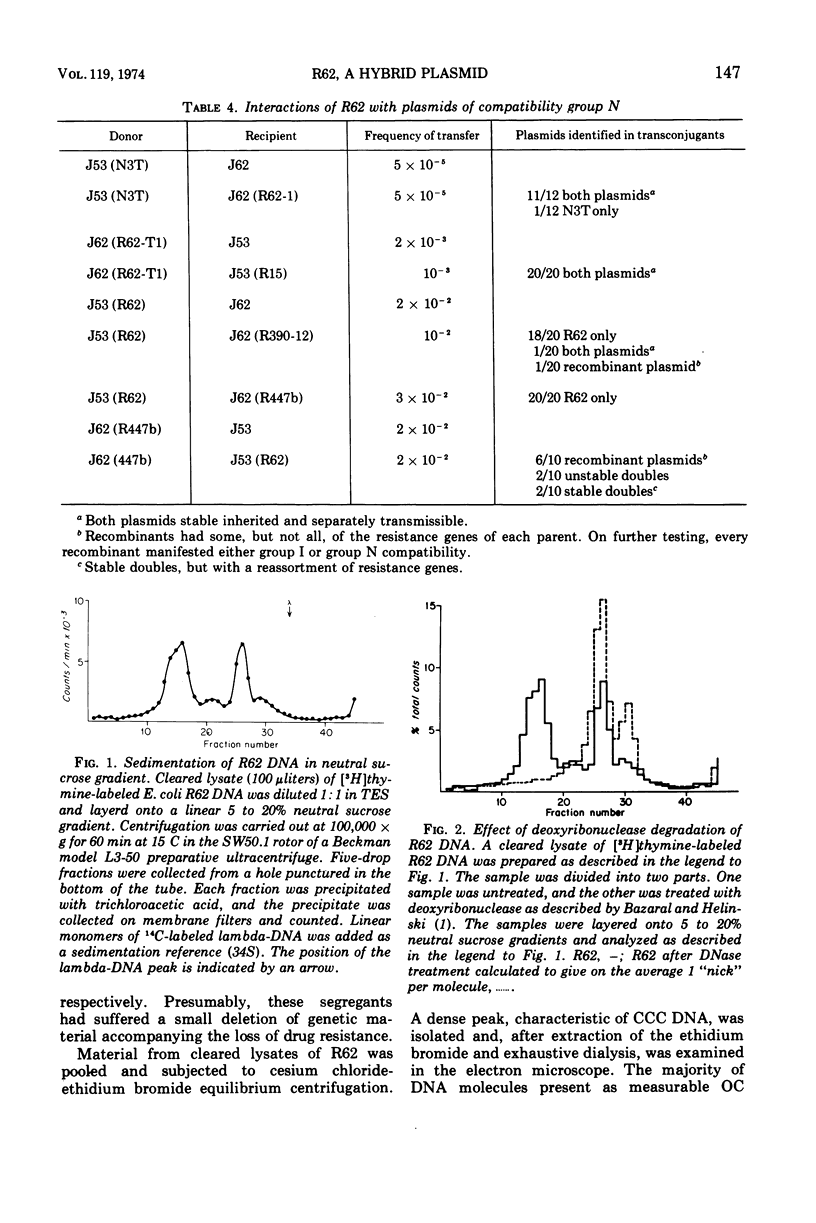
R62, a naturally occurring R factor, was shown to be a single deoxyribonucleic acid molecule composed of polynucleotide sequences typical of I group plasmids and also sequences typical of the N group. It determined I pili and belonged to the Iα compatibility group. Although compatible with plasmids of group N, R62 showed complex genetic reactions with N plasmids which are described and interpreted. It is concluded that R62 was the product of illegitimate recombination between an I group and an N group plasmid.
Full text
PDF





Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bazaral M., Helinski D. R. Characterization of multiple circular DNA forms of colicinogenic factor E-1 from Proteus mirabilis. Biochemistry. 1968 Oct;7(10):3513–3520. doi: 10.1021/bi00850a028. [DOI] [PubMed] [Google Scholar]
- Clewell D. B., Helinski D. R. Supercoiled circular DNA-protein complex in Escherichia coli: purification and induced conversion to an opern circular DNA form. Proc Natl Acad Sci U S A. 1969 Apr;62(4):1159–1166. doi: 10.1073/pnas.62.4.1159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clowes R. C. Molecular structure of bacterial plasmids. Bacteriol Rev. 1972 Sep;36(3):361–405. doi: 10.1128/br.36.3.361-405.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coetzee J. N., Datta N., Hedges R. W. R factors from Proteus rettgeri. J Gen Microbiol. 1972 Oct;72(3):543–552. doi: 10.1099/00221287-72-3-543. [DOI] [PubMed] [Google Scholar]
- Cohen S. N., Miller C. A. Non-chromosomal antibiotic resistance in bacteria. II. Molecular nature of R-factors isolated from Proteus mirabilis and Escherichia coli. J Mol Biol. 1970 Jun 28;50(3):671–687. doi: 10.1016/0022-2836(70)90092-6. [DOI] [PubMed] [Google Scholar]
- Datta N., Hedges R. W. An I pilus-determining R factor with anomalous compatibility properties, mobilizing a gentamicin-resistance plasmid. J Gen Microbiol. 1973 Jul;77(1):11–17. doi: 10.1099/00221287-77-1-11. [DOI] [PubMed] [Google Scholar]
- Datta N., Hedges R. W. Compatibility groups among fi - R factors. Nature. 1971 Nov 26;234(5326):222–223. doi: 10.1038/234222a0. [DOI] [PubMed] [Google Scholar]
- Datta N., Hedges R. W., Shaw E. J., Sykes R. B., Richmond M. H. Properties of an R factor from Pseudomonas aeruginosa. J Bacteriol. 1971 Dec;108(3):1244–1249. doi: 10.1128/jb.108.3.1244-1249.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Datta N., Kontomichalou P. Penicillinase synthesis controlled by infectious R factors in Enterobacteriaceae. Nature. 1965 Oct 16;208(5007):239–241. doi: 10.1038/208239a0. [DOI] [PubMed] [Google Scholar]
- Davies J., Brzezinska M., Benveniste R. The problems of drug-resistant pathogenic bacteria. R factors: biochemical mechanisms of resistance to aminoglycoside antibiotics. Ann N Y Acad Sci. 1971 Jun 11;182:226–233. doi: 10.1111/j.1749-6632.1971.tb30659.x. [DOI] [PubMed] [Google Scholar]
- Falkow S., Tompkins L. S., Silver R. P., Guerry P., Le Blanc D. J. The problems of drug-resistant pathogenic bacteria. The replication of R-factor DNA in Escherichia coli K-12 following conjugation. Ann N Y Acad Sci. 1971 Jun 11;182:153–171. doi: 10.1111/j.1749-6632.1971.tb30654.x. [DOI] [PubMed] [Google Scholar]
- Guerry P., Falkow S. Polynucleotide sequence relationships among some bacterial plasmids. J Bacteriol. 1971 Jul;107(1):372–374. doi: 10.1128/jb.107.1.372-374.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hedges R. W., Datta N., Coetzee J. N., Dennison S. R factors from Proteus morganii. J Gen Microbiol. 1973 Aug;77(2):249–259. doi: 10.1099/00221287-77-2-249. [DOI] [PubMed] [Google Scholar]
- Hedges R. W., Datta N., Kontomichalou P., Smith J. T. Molecular specificities of R factor-determined beta-lactamases: correlation with plasmid compatibility. J Bacteriol. 1974 Jan;117(1):56–62. doi: 10.1128/jb.117.1.56-62.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hedges R. W., Datta N. Plasmids determining I pili constitute a compatibility complex. J Gen Microbiol. 1973 Jul;77(1):19–25. doi: 10.1099/00221287-77-1-19. [DOI] [PubMed] [Google Scholar]
- Hedges R. W. Phenotypic characterization of fi- R factors determining the restriction and modification hsp II specificity. Mol Gen Genet. 1972;115(3):225–233. doi: 10.1007/BF00268886. [DOI] [PubMed] [Google Scholar]
- Hedges R. W. Resistance to spectinomycin determined by R factors of various compatibility groups. J Gen Microbiol. 1972 Sep;72(2):407–409. doi: 10.1099/00221287-72-2-407. [DOI] [PubMed] [Google Scholar]
- Jenkins P. H., Drabble W. T. -lactamases of R factors derived from Shigella and Salmonella strains. J Bacteriol. 1971 Oct;108(1):159–165. doi: 10.1128/jb.108.1.159-165.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LENNOX E. S. Transduction of linked genetic characters of the host by bacteriophage P1. Virology. 1955 Jul;1(2):190–206. doi: 10.1016/0042-6822(55)90016-7. [DOI] [PubMed] [Google Scholar]
- Lawn A. M., Meynell G. G., Meynell E., Datta N. Sex pili and the classification of sex factors in the enterobacteriaceae. Nature. 1967 Oct 28;216(5113):343–346. doi: 10.1038/216343a0. [DOI] [PubMed] [Google Scholar]
- LeBlanc D. J., Falkow S. Studies on superinfection immunity among transmissible plasmids in Escherichia coli. J Mol Biol. 1973 Mar 15;74(4):689–701. doi: 10.1016/0022-2836(73)90057-0. [DOI] [PubMed] [Google Scholar]
- Meynell E. Pseudo-fi + I-like sex factors, R62(I), selective for increased pilus synthesis. J Bacteriol. 1973 Jan;113(1):502–503. doi: 10.1128/jb.113.1.502-503.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meynell G. G., Lawn A. M. Filamentous phages specific for the I sex factor. Nature. 1968 Mar 23;217(5134):1184–1186. doi: 10.1038/2171184a0. [DOI] [PubMed] [Google Scholar]
- Nisioka T., Mitani M., Clowes R. Composite circular forms of R factor deoxyribonucleic acid molecules. J Bacteriol. 1969 Jan;97(1):376–385. doi: 10.1128/jb.97.1.376-385.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Romero E., Meynell E. Covert fi- R factors in fi+ R+ strains of bacteria. J Bacteriol. 1969 Feb;97(2):780–786. doi: 10.1128/jb.97.2.780-786.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silver R. P., Falkow S. Specific labeling and physical characterization of R-factor deoxyribonucleic acid in Escherichia coli. J Bacteriol. 1970 Oct;104(1):331–339. doi: 10.1128/jb.104.1.331-339.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watanabe T., Sakaizumi S., Furuse C. Superinfection with R factors by transduction in Escherichia coli and Salmonella typhimurium. J Bacteriol. 1968 Nov;96(5):1796–1802. doi: 10.1128/jb.96.5.1796-1802.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Young E. T., 2nd, Sinsheimer R. L. Vegetative bacteriophage lambda-DNA. II. Physical characterization and replication. J Mol Biol. 1967 Nov 28;30(1):165–200. doi: 10.1016/0022-2836(67)90251-3. [DOI] [PubMed] [Google Scholar]


