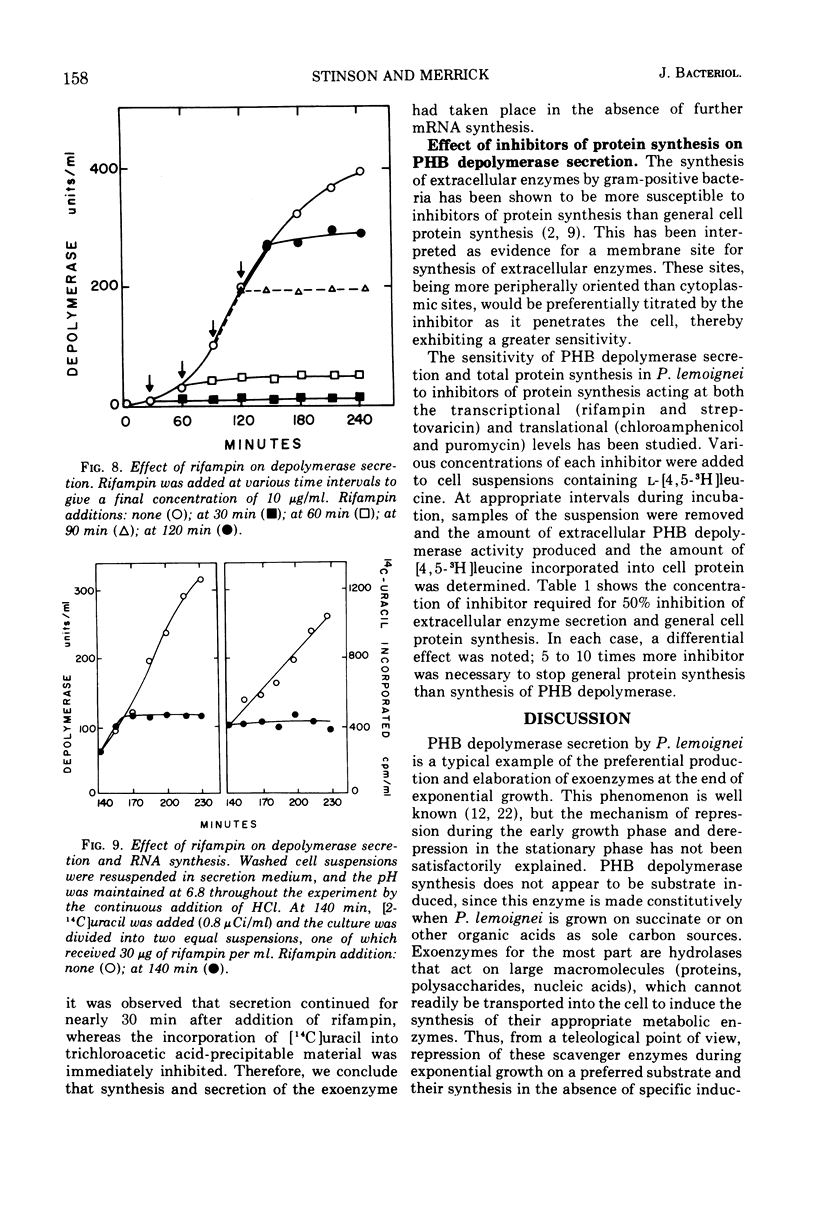
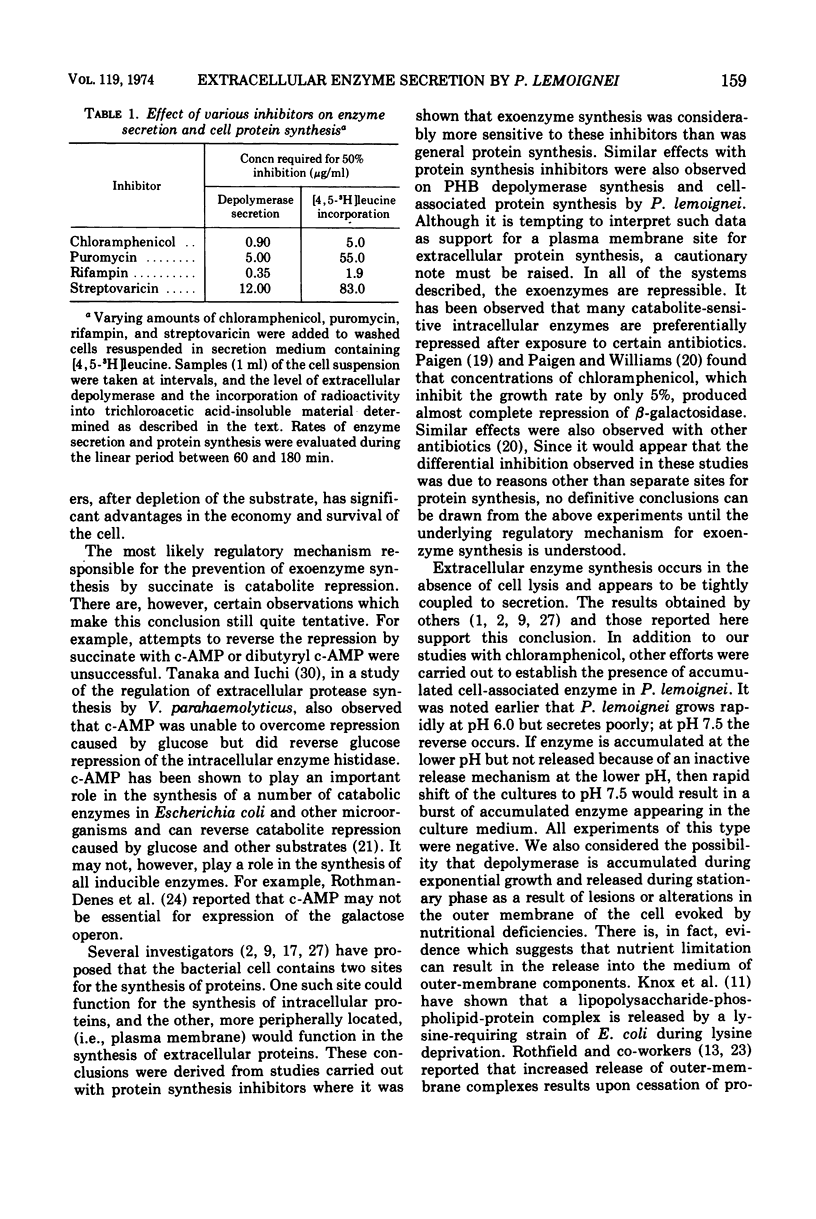
Abstract
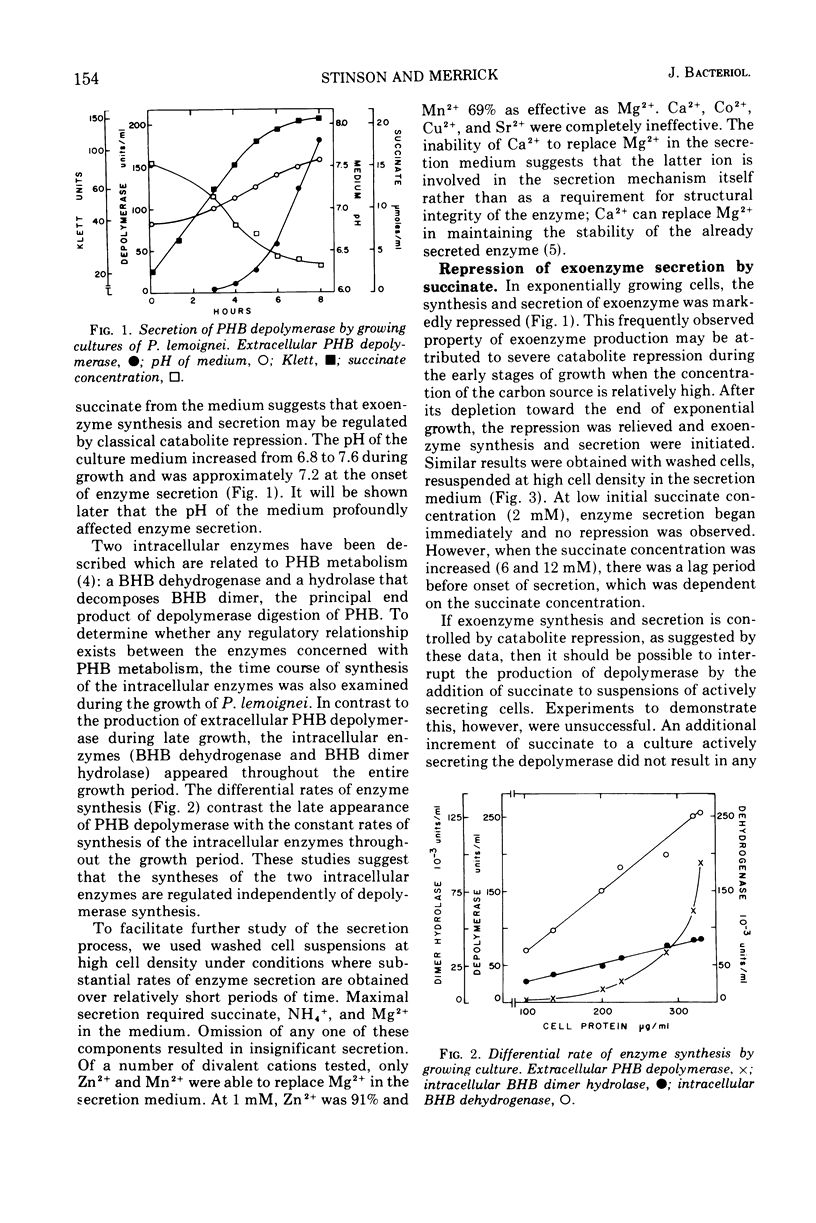
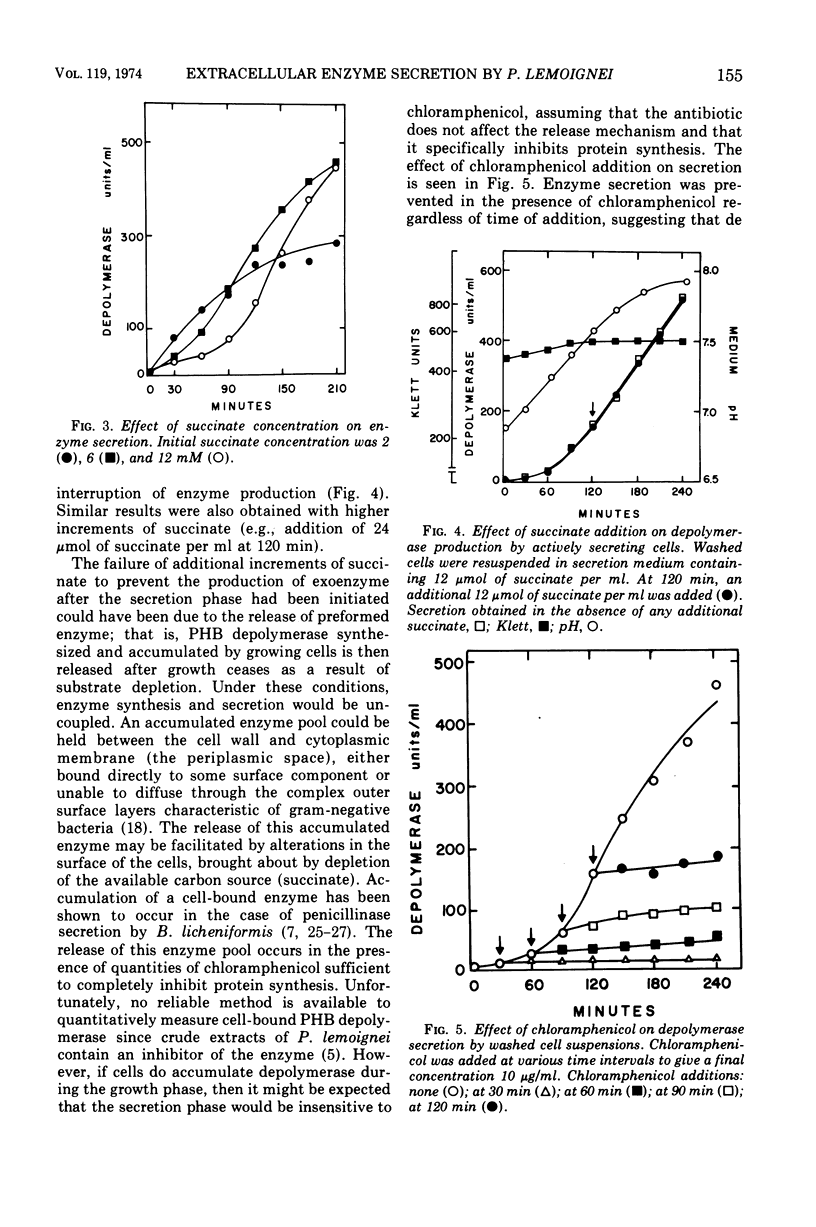
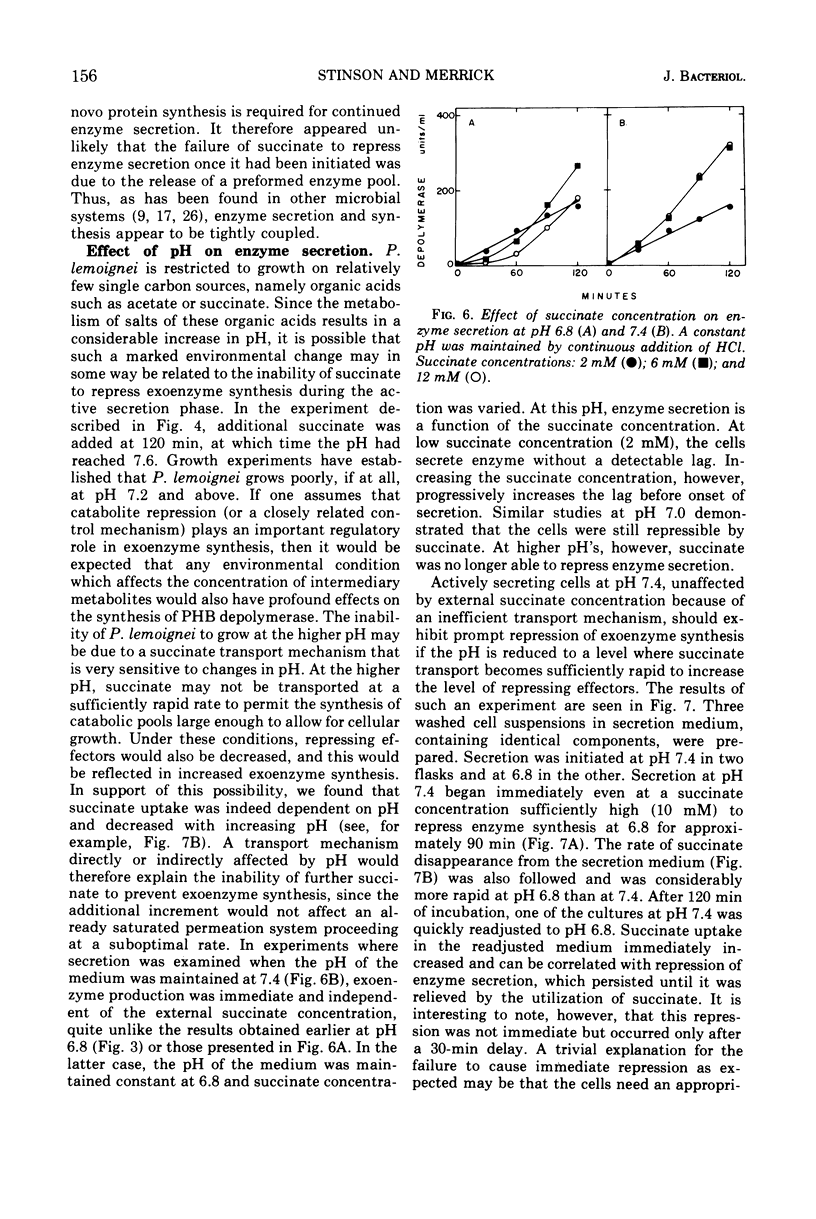
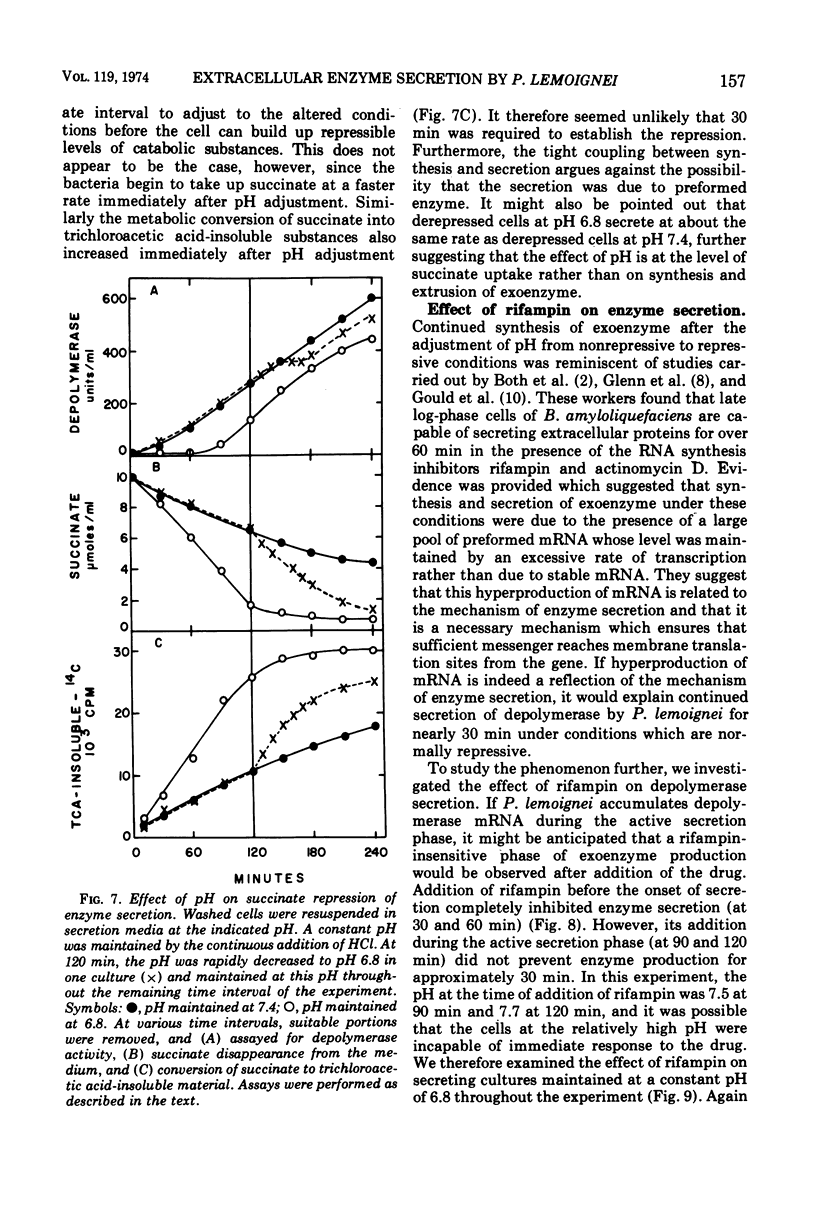
The ability of succinate to repress the secretion of Pseudomonas lemoignei poly-β-hydroxybutyrate depolymerase was a function of pH. Repression only occurred when the pH of the medium was 7.0 or less. At a higher pH, lack of sensitivity to succinate concentration may have been due to a limited ability to transport succinate. Actively secreting cultures (at pH 7.4) continued to secrete enzyme for approximately 30 min after the pH was rapidly decreased to pH 6.8, even though sufficient succinate was present to repress enzyme synthesis. Similarly, after the addition of rifampin to secreting cultures, there was a 30-min delay before secretion was inhibited. Evidence is presented which suggests that continued secretion may be the result of depolymerase messenger ribonucleic acid accumulation within the cells. Studies with chloramphenicol indicated that de novo protein synthesis is necessary for the secretion of poly-β-hydroxybutyrate depolymerase and that exoenzyme is not released from a preformed pool. Studies with various inhibitors of protein synthesis indicated that synthesis of exoenzyme is 5 to 10 times more susceptible to inhibition than is the synthesis of cell-associated proteins.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bettinger G. E., Lampen J. O. Evidence for the extrusion of an incompletely folded form of penicillinase during secretion by protoplasts of Bacillus licheniformis 749-C. Biochem Biophys Res Commun. 1971 Apr 2;43(1):200–206. doi: 10.1016/s0006-291x(71)80107-9. [DOI] [PubMed] [Google Scholar]
- Both G. W., McInnes J. L., Hanlon J. E., May B. K., Elliott W. H. Evidence for an accumulation of messenger RNA specific for extracellular protease and its relevance to the mechanism of enzyme secretion in bacteria. J Mol Biol. 1972 Jun 20;67(2):199–217. doi: 10.1016/0022-2836(72)90236-7. [DOI] [PubMed] [Google Scholar]
- Delafield F. P., Cooksey K. E., Doudoroff M. beta-Hydroxybutyric dehydrogenase and dimer hydrolase of Pseudomonas lemoignei. J Biol Chem. 1965 Oct;240(10):4023–4028. [PubMed] [Google Scholar]
- Delafield F. P., Doudoroff M., Palleroni N. J., Lusty C. J., Contopoulos R. Decomposition of poly-beta-hydroxybutyrate by pseudomonads. J Bacteriol. 1965 Nov;90(5):1455–1466. doi: 10.1128/jb.90.5.1455-1466.1965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghosh B. K., Sargent M. G., Lampen J. O. Morphological phenomena associated with penicillinase induction and secretion in Bacillus licheniformis. J Bacteriol. 1968 Oct;96(4):1314–1328. doi: 10.1128/jb.96.4.1314-1328.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glenn A. R., Both G. W., McInnes J. L., May B. K., Elliott W. H. Dynamic state of the messenger RNA pool specific for extracellular protease in Bacillus amyloliquefaciens: its relevance to the mechanism of enzyme secretion. J Mol Biol. 1973 Jan 10;73(2):221–230. doi: 10.1016/0022-2836(73)90325-2. [DOI] [PubMed] [Google Scholar]
- Glew R. H., Heath E. C. Studies on the extracellular alkaline phosphatase of Micrococcus sodonensis. II. Factors affecting secretion. J Biol Chem. 1971 Mar 25;246(6):1566–1574. [PubMed] [Google Scholar]
- Gould A. R., May B. K., Elliott W. H. Accumulation of messenger RNA for extracellular enzymes as a general phenomenon in Bacillus amyloiquefaciens. J Mol Biol. 1973 Jan 10;73(2):213–219. doi: 10.1016/0022-2836(73)90324-0. [DOI] [PubMed] [Google Scholar]
- Knox K. W., Cullen J., Work E. An extracellular lipopolysaccharide-phospholipid-protein complex produced by Escherichia coli grown under lysine-limiting conditions. Biochem J. 1967 Apr;103(1):192–201. doi: 10.1042/bj1030192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Lopes J., Gottfried S., Rothfield L. Leakage of periplasmic enzymes by mutants of Escherichia coli and Salmonella typhimurium: isolation of "periplasmic leaky" mutants. J Bacteriol. 1972 Feb;109(2):520–525. doi: 10.1128/jb.109.2.520-525.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lusty C. J., Doudoroff M. Poly-beta-hydroxybutyrate depolymerases of Pseudomonas lemoignei. Proc Natl Acad Sci U S A. 1966 Sep;56(3):960–965. doi: 10.1073/pnas.56.3.960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MACRAE R. M., WILKINSON J. F. Poly-beta-hyroxybutyrate metabolism in washed suspensions of Bacillus cereus and Bacillus megaterium. J Gen Microbiol. 1958 Aug;19(1):210–222. doi: 10.1099/00221287-19-1-210. [DOI] [PubMed] [Google Scholar]
- May B. K., Elliott W. H. Characteristics of extracellular protease formation by Bacillus subtilis and its control by amino acid repression. Biochim Biophys Acta. 1968 May 21;157(3):607–615. doi: 10.1016/0005-2787(68)90158-5. [DOI] [PubMed] [Google Scholar]
- Neu H. C., Heppel L. A. The release of enzymes from Escherichia coli by osmotic shock and during the formation of spheroplasts. J Biol Chem. 1965 Sep;240(9):3685–3692. [PubMed] [Google Scholar]
- PAIGEN K. CHANGES IN THE INDUCIBILITY OF GALACTOKINASE AND BETA-GALACTOSIDASE DURING INHIBITION OF GROWTH IN ESCHERICHIA COLI. Biochim Biophys Acta. 1963 Oct 1;77:318–328. doi: 10.1016/0006-3002(63)90502-x. [DOI] [PubMed] [Google Scholar]
- Pastan I., Perlman R. Cyclic adenosine monophosphate in bacteria. Science. 1970 Jul 24;169(3943):339–344. doi: 10.1126/science.169.3943.339. [DOI] [PubMed] [Google Scholar]
- Rothfield L., Pearlman-Kothencz M. Synthesis and assembly of bacterial membrane components. A lipopolysaccharide-phospholipid-protein complex excreted by living bacteria. J Mol Biol. 1969 Sep 28;44(3):477–492. doi: 10.1016/0022-2836(69)90374-x. [DOI] [PubMed] [Google Scholar]
- Rothman-Denes L. B., Hesse J. E., Epstein W. Role of cyclic adenosine 3',5'-monophosphate in the in vivo expression of the galactose operon of Escherichia coli. J Bacteriol. 1973 Jun;114(3):1040–1044. doi: 10.1128/jb.114.3.1040-1044.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SHUSTER C. W., DOUDOROFF M. A cold-sensitive D(-) beta-hydroxybutyric acid dehydrogenase from Rhodospirillum rubrum. J Biol Chem. 1962 Feb;237:603–607. [PubMed] [Google Scholar]
- Sargent M. G., Ghosh B. K., Lampen J. O. Characteristics of penicillinase secretion by growing cells and protoplasts of Bacillus licheniformis. J Bacteriol. 1969 Feb;97(2):820–826. doi: 10.1128/jb.97.2.820-826.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sargent M. G., Ghosh B. K., Lampen J. O. Localization of cell-bound penicillinase in Bacillus licheniformis. J Bacteriol. 1968 Oct;96(4):1329–1338. doi: 10.1128/jb.96.4.1329-1338.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sargent M. G., Lampen J. O. A mechanism for penicillinasesecretion in Bacillus licheniformis. Proc Natl Acad Sci U S A. 1970 Apr;65(4):962–969. doi: 10.1073/pnas.65.4.962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanaka S., Iuchi S. Induction and repression of an extracellular proteinase in Vibrio parahaemolyticus. Biken J. 1971 Jun;14(2):81–96. [PubMed] [Google Scholar]


