Abstract
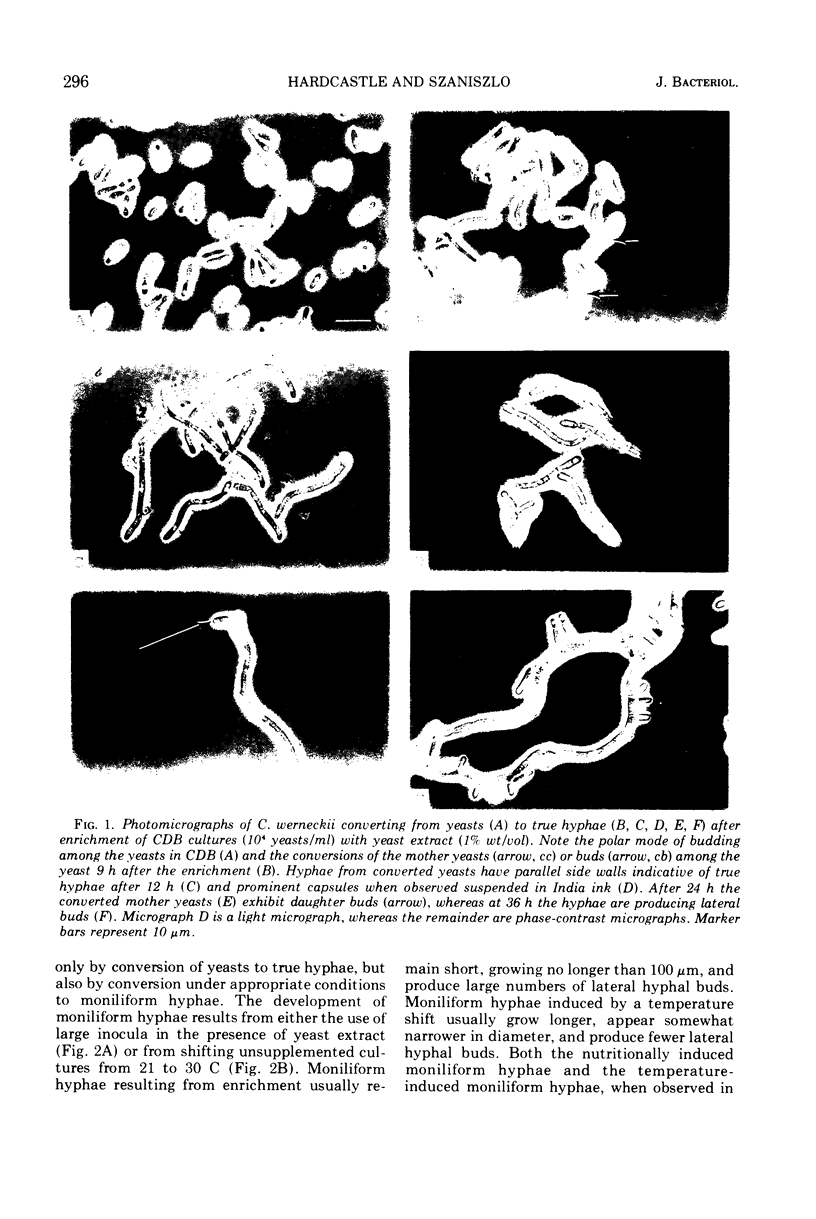
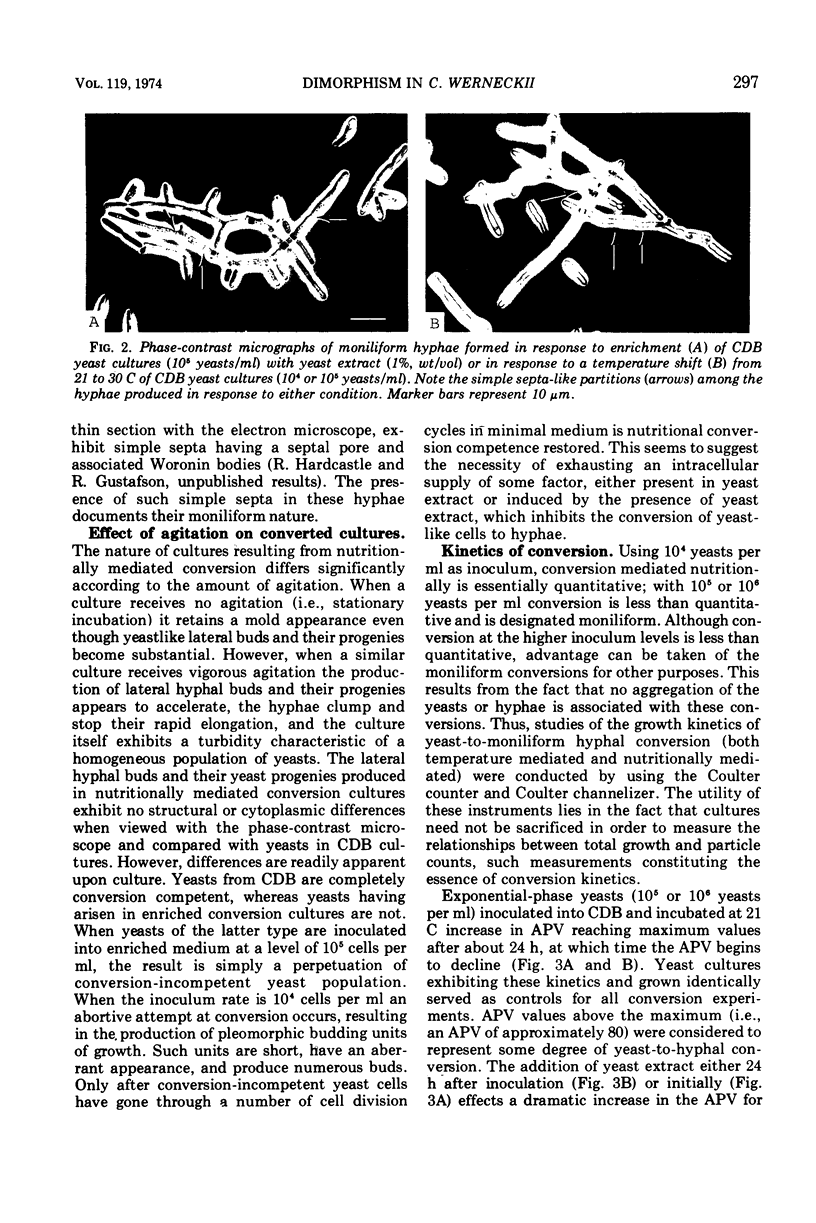
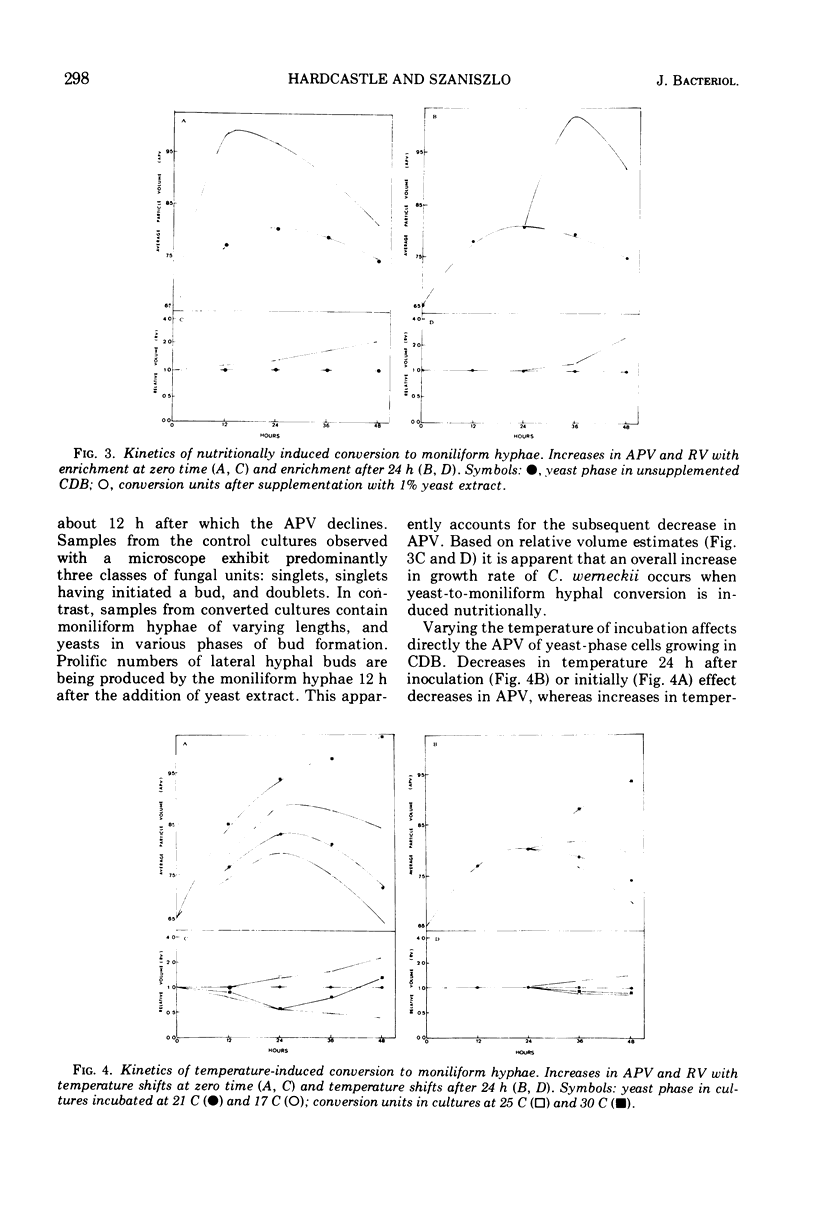
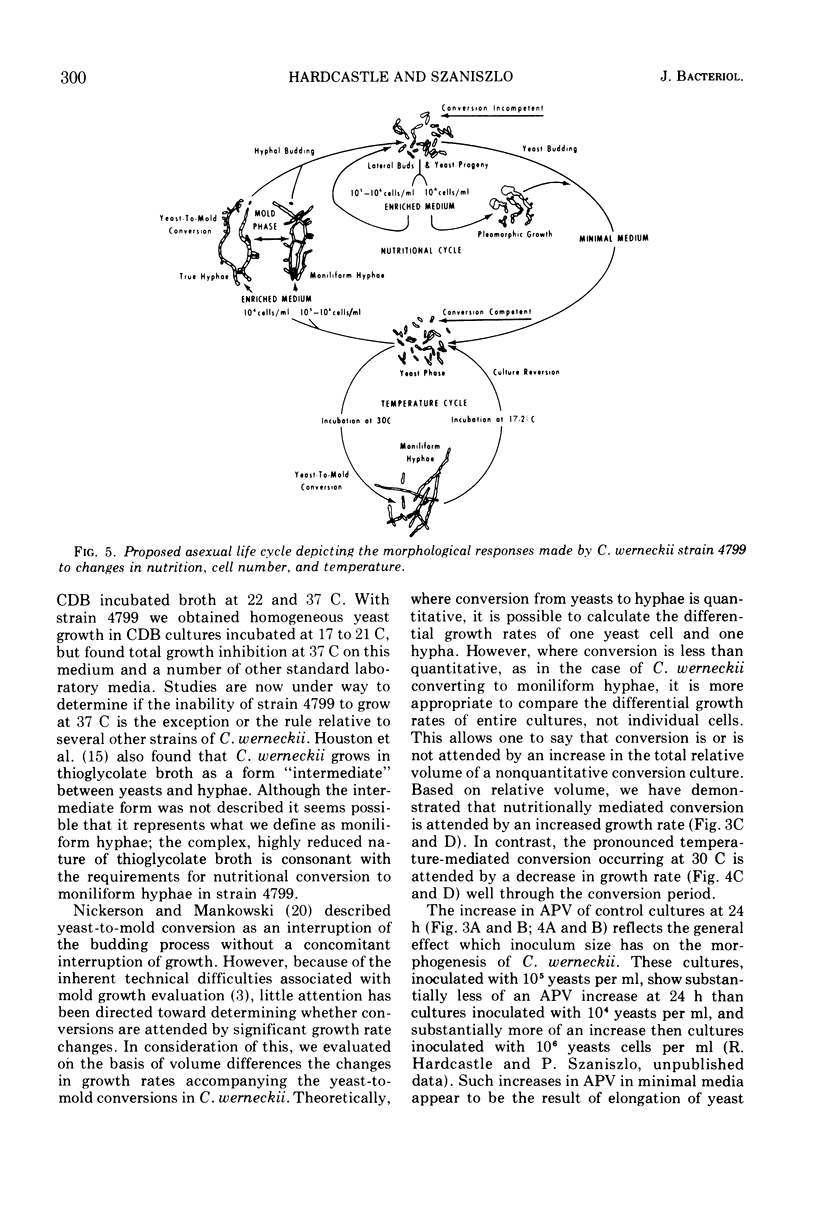
Yeast forms of the dimorphic fungus Cladosporium werneckii grow by polar budding and yield a homogeneous yeast phase when cultured at 21 C in an agitated sucrose-salts medium (Czapek-Dox broth). Yeast extract enrichment of such a yeast phase consisting of 104 yeasts per ml induces a quantitative conversion of the yeasts to true hyphae. This conversion is not mediated by a transition cell and is often attended by capsule formation. When 105 or 106 yeasts per ml receive enrichment, a nonquantitative conversion to moniliform hyphae is effected and no capsule formation is observed. Rapid agitation compared to slow agitation or stationary incubation of the nutritionally mediated conversion cultures greatly accelerates the production of lateral hyphal buds or their yeast progenies. These cells appear incapable of undergoing nutritional conversion to hyphae, but instead must grow for several generations in the unenriched sucrose-salts medium to restore conversion competence. Temperature shifts affect directly the morphology and morphogenesis of the yeast in unenriched medium; at 17 C yeasts are smaller and more ovoid than at 21 C, and at 30 C marked conversion of yeasts to moniliform hyphae occurs. A methodology employing the Coulter counter and Coulter channelizer provides evidence that direct correlations do not always exist between the optimum conditions for the growth of C. werneckii and the optimum conditions for its yeast-to-mold conversion.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BARTNICKI-GARCIA S., NICKERSON W. J. Induction of yeast-like development in Mucor by carbon dioxide. J Bacteriol. 1962 Oct;84:829–840. doi: 10.1128/jb.84.4.829-840.1962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CARRION A. L. Yeastlike dematiaceous fungi infecting the human skin; special reference to so-called Hormiscium dermatitidis. Arch Derm Syphilol. 1950 Jun;61(6):996–1009. doi: 10.1001/archderm.1950.01530130114017. [DOI] [PubMed] [Google Scholar]
- Campbell C. C. Reverting Histoplasma capsulatum to the Yeast Phase. J Bacteriol. 1947 Aug;54(2):263–264. [PMC free article] [PubMed] [Google Scholar]
- Carbonell L. M. Ultrastructure of dimorphic transformation in paracoccidioides brasiliensis. J Bacteriol. 1969 Nov;100(2):1076–1082. doi: 10.1128/jb.100.2.1076-1082.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Castellani A. Tinea nigra. Some remarks and annotations, chiefly historical. Mycopathol Mycol Appl. 1966 Dec 1;30(3):193–199. doi: 10.1007/BF02053147. [DOI] [PubMed] [Google Scholar]
- Conant N. F. Cultural Study of the Life-Cycle of Histoplasma capsulatum Darling 1906. J Bacteriol. 1941 May;41(5):563–579. doi: 10.1128/jb.41.5.563-579.1941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DROUHET E., MARIAT F. Etude des facteurs déterminant le développement de la phase levure de Sporotrichum schencki. Ann Inst Pasteur (Paris) 1952 Oct;83(4):506–514. [PubMed] [Google Scholar]
- Grove S. N., Oujezdsky K. B., Szaniszlo P. J. Budding in the dimorphic fungus Phialophora dermatitidis. J Bacteriol. 1973 Jul;115(1):323–329. doi: 10.1128/jb.115.1.323-329.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HOWARD D. H. The morphogenesis of the parasitic forms of dimorphic fungi. A review. Mycopathol Mycol Appl. 1962 Oct 10;18:127–139. doi: 10.1007/BF02055153. [DOI] [PubMed] [Google Scholar]
- Houston M. R., Meyer K. H., Thomas N., Wolf F. T. Dimorphism in Cladosporium werneckii. Sabouraudia. 1969 Oct;7(3):195–198. [PubMed] [Google Scholar]
- Itani Z. Tinea nigra palmaris. Mykosen. 1972 Jan 1;15(1):27–34. doi: 10.1111/j.1439-0507.1972.tb02424.x. [DOI] [PubMed] [Google Scholar]
- KURUNG J. M., YEGIAN D. Medium for maintenance and conversion of Histoplasma capsulatum to yeast like phase. Am J Clin Pathol. 1954 Apr;24(4):505–508. doi: 10.1093/ajcp/24.4_ts.505. [DOI] [PubMed] [Google Scholar]
- Levine S., Ordal Z. J. Factors Influencing the Morphology of Blastomyces dermatitidis. J Bacteriol. 1946 Dec;52(6):687–694. doi: 10.1128/jb.52.6.687-694.1946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nielsen H. S., Jr, Conant N. F. Practical evaluation of antigenic relationships of yeastlike dematiaceous fungi. Sabouraudia. 1967 Jun;5(4):283–294. doi: 10.1080/00362176785190541. [DOI] [PubMed] [Google Scholar]
- Oujezdsky K. B., Grove S. N., Szaniszlo P. J. Morphologica and structural changes during the yeast-to mold conversion of Phialophora dermatitidis. J Bacteriol. 1973 Jan;113(1):468–477. doi: 10.1128/jb.113.1.468-477.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- PINE L. Studies on the growth of Histoplasma capsulatum. I. Growth of the yeast phase in liquid media. J Bacteriol. 1954 Dec;68(6):671–679. doi: 10.1128/jb.68.6.671-679.1954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SCHERR G. H. Studies on the dimorphism of Histoplasma capsulatum. I. The roles of -SH groups and incubation temperature. Exp Cell Res. 1957 Feb;12(1):92–107. doi: 10.1016/0014-4827(57)90296-3. [DOI] [PubMed] [Google Scholar]




