Abstract
Tyrosine phosphorylation has been shown to be an important modulator of synaptic transmission in both vertebrates and invertebrates. Such findings hint toward the existence of extracellular ligands capable of activating this widely represented signaling mechanism at or close to the synapse. Examples of such ligands are the peptide growth factors which, on binding, activate receptor tyrosine kinases. To gain insight into the physiological consequences of receptor tyrosine kinase activation in squid giant synapse, a series of growth factors was tested in this preparation. Electrophysiological, pharmacological, and biochemical analysis demonstrated that nerve growth factor (NGF) triggers an acute and specific reduction of the postsynaptic potential amplitude, without affecting the presynaptic spike generation or presynaptic calcium current. The NGF target is localized at a postsynaptic site and involves a new TrkA-like receptor. The squid receptor crossreacts with antibodies generated against mammalian TrkA, is tyrosine phosphorylated in response to NGF stimulation, and is blocked by specific pharmacological inhibitors. The modulation described emphasizes the important role of growth factors on invertebrate synaptic transmission.
Keywords: neurotrophins and neurotrophis/synaptic transmission/presynaptic/phosphorylation/autophosphorylation
Neurotrophins are a family of neurotrophic substances that includes nerve growth factor (NGF), brain-derived growth factor (BDNF), and the NT3, 4/5, 6, and 7 neurotrophins (1, 2). These molecules are major regulators of neuronal differentiation, survival, and repair, but they also exert short- and long-term effects on central nervous system and peripheral nervous system synaptic physiology in vertebrates (3, 4). Neurotrophins interact with two types of membrane proteins, the Trk family of receptor tyrosine kinase (RTK) and the low-affinity p75 receptor (the latter has not been determined for NT7). Receptors of the Trk family display neurotrophin selectivity, thus NGF, NT6, and NT7 signal preferentially through TrkA, while BDNF and NT3 are the ligands for TrkB and TrkC, respectively. In turn, NT3 and NT4/5 can activate both TrkA and TrkB (1, 5). Ligand binding to Trk receptors results in, (i) dimerization of the receptor molecules, followed by autophosphorylation of certain tyrosine residues in cytoplasmic domains (6), and (ii) activation of a signaling cascade, either by activation of protein tyrosine kinase directly or by serving as binding sites for downstream signaling proteins containing Src-homology 2 or phosphotyrosine binding domains. In recent years, these themes have been studied in detail at both molecular and functional levels (7–10).
Whereas it has been proposed that invertebrates lack neurotrophin-like molecules (11), neurotrophins may be involved in invertebrate neurite outgrowth (12), synapse formation (13), synaptic plasticity (14), and calcium channel modulation (15). Moreover, a putative neurotrophic factor, termed cysteine-rich neurotrophin, was recently identified in the mollusk Lymnaea stagnalis. Cysteine-rich neurotrophin, which does not have significant sequence homology to vertebrate neurotrophins, does bind p75 receptor and potentiates voltage-dependent calcium currents (16). In addition, a new neurotrophin receptor termed Ltrk, an RTK similar to TrkC that binds NT3 specifically (17), has been identified in the same mollusk. Because protein tyrosine phosphorylation is an important synaptic transmission modulator at the squid giant synapse (18), we tested the effects of peptide growth factors that may activate endogenous RTKs at this synapse.
The present set of experiments provides the first example of a functional TrkA-like receptor in an invertebrate synapse, as well as its physiological role and molecular pharmacological profile. This finding and those in Lymnaea further sharpen the debate regarding the evolutionary origin of neurotrophins and their receptors, as well as their physiological role during embryonic and adult life.
MATERIALS AND METHODS
Electrophysiology.
Stellate ganglia from the squid (Loligo peallei) were isolated from the mantle (19) and placed in oxygenated artificial seawater buffer with Tris [423 mM NaCl, 8, 3 mM KCl, 10 mM CaCl2, 50 mM MgCl2, 2 mM Tris (pH 7.2)], and 0.001% mM H2O2) and maintained at room temperature. Thirty-six successfully isolated giant synapses were used in the electrophysiological study. The presynaptic terminal was impaled with two sharp microelectrodes (one to inject current and the other to measure voltage). The postsynaptic potential was measured with a third microelectrode at the site of the synaptic junction. Microinjection was imaged by using a fluorescent dye (0.001% dextran fluorescein). Sodium and potassium currents during the voltage-clamp experiments were blocked with 1 mM tetrodotoxin and 5 mM 3- or 4-aminopyridine, respectively. The voltage-clamp steps were delivered from a holding potential of −70 mV.
Pharmacology.
βb-NGF (Calbiochem), HRG-βb1 [epidermal growth factor (EGF) domain], EGF (R & D Systems), hNT-3 and hBDNF, and acidic fibroblast growth factor (aFGF) (Alamone Labs, Jerusalem). All growth factors were human version and were reconstituted following the indications of manufacturers in a stock solution of 20 μg/ml and were stored at −20°C. At time of use, the desired concentrations were adjusted with regular sea water. Growth factors were applied directly on the synapse by means of an extracellular electrode by using pressure pulses. Genistein (Research Biochemicals, Natick, MA), SU5402, SU4984 (kind gift from Sugen) and KT 252a (Calbiochem) were prepared in 1,000× stock in dimethyl sulfoxide (Sigma) and were diluted to the final concentration in the appropriate solution.
Cell Lines.
PC12 were grown to 70% confluence (100-mm dishes) in DMEM with 10% fetal calf serum and 10% horse serum. PC12 cells overexpressing TrkA receptors (PC12/TrkA) were described previously (20). Parental PC12 cells were from the same batch as those used for the transfection.
Antibodies.
Antiphosphotyrosine antibodies were described previously (21). Anti-Trk antibodies (Trk C-14) were purchased from Santa Cruz Biotechnology. Antibodies against the C2A domain of the squid version of syntaxin (22) were a kind gift of Mitsunori Fukuda.
Immunoblotting and Immunoprecipitation.
PC12 cells (untransfected and transfected) were lysed for 30 min at 4°C in 50 mM Tris⋅HCl (pH 7.4)/150 mM NaCl/1 mM EDTA/1 mM EGTA/1 mM NaVO3/1% Triton X-100/protease inhibitors as suggested by the manufacturer (Sigma) (lysis buffer). PC12 cells overexpressing TrkA were stimulated with 100 ng/ml of NGF for 15 min at 37°C in regular DMEM and solubilized with lysis buffer, then were immunoprecipitated with anti-Trk antibodies and protein A sepharose beads (Sigma). Protein concentration was determined by Bradford assay (Bio-Rad), and equal amounts were loaded onto SDS/PAGE, were run, and were transferred to nitrocellulose by standard methods. Blots were probed with the appropriate antibody (see figure legends). Immunoblots and immunoprecipitations were done as described in Dikic et al. (23). Trk tyrosine phosphorylation in NGF-stimulated PC12/trkA cells was detected by blotting with antiphosphotyrosine-specific antibodies as described in Kouhara et al. (21). A total of 300 freshly isolated squid stellate ganglia, a preparation identical to that used in the electrophysiological studies, were homogenized (30 per procedure) in a solution containing 0.32 M sucrose/5 mM Tris⋅HCl (pH 7.4)/protease inhibitor mixture (Sigma)/1 mM NaVO3. Homogenate was centrifuged at 700 × g for 10 min at 4°C. The supernatant was centrifuged at 27,000 × g for 40 min at 4°C. The 27,000 × g pellet was resuspended in a solution containing 5 mM Tris/1 mM EDTA/protease inhibitor cocktail (Sigma)/1 mM NaVO3 (pH 8.0), was homogenized again and was centrifuged 80,000 × g for 30 min at 4°C. The pellet was resuspended in a solution containing 200 mM KCl/10 mM Hepes protease inhibitor mixture (Sigma)/1 mM NaVO3 (pH 7.4) and then mixed 1:3 with a 3% triton lysis buffer as described above. Immunoprecipitation and immunoblotting were done as described previously. In some experiments, the stellate ganglia were maintained in artificial sea water, were incubated with 200 nM KT252a for 15 min, then were stimulated with 200 ng/ml of NGF for 15 min and were subjected to the same protocol, but were immunoblotted with antiphosphotyrosine antibodies. In some cases, stellate ganglia were solubilized after homogenization in 0.32 M sucrose with similar results. Anti-Trk antibodies were used at a final concentration of 4 μg/ml and syntaxin antibodies were used in a 1:100 dilution from the original batch.
RESULTS
Acute Inhibition of Synaptic Transmission by a NGF-Activated Tyrosine Kinase.
Pre- and postsynaptic potentials recorded simultaneously under current clamp configuration were evoked by preterminal electrical stimulation (19). Synaptic transmission triggered once every 3 min was markedly reduced by extracellular application of 100 ng/ml of NGF (n = 6). In the experiment illustrated in Fig. 1A, a clear reduction of the rate of rise of the excitatory postsynaptic potential was observable at 10 min after application of NGF. The postsynaptic potential became subthreshold for action potential generation after 20 min and continued to decrease in amplitude over the next 40 min. No significant effect was observed in the amplitude or duration of the presynaptic action potential (Fig. 1A). Similar results were obtained in the other five experiments where this paradigm was implemented. Because NGF in vertebrates can signal either through activation of an RTK (TrkA) or through p75, the postsynaptic terminal was intracellularly microinjected with the tyrosine kinase inhibitor genistein (10 μM) under direct visualization (see Methods). Thus, as shown in Fig. 2A, local superfusion with 100 ng/ml NGF on genistein preinjected cells produced no significant change in synaptic transmission (n = 4). These results demonstrate that tyrosine kinase activation is a prerequisite for NGF modulation and eliminates the possibility of a nonspecific block, and they imply that the target of NGF-activated kinase is postsynaptically located.
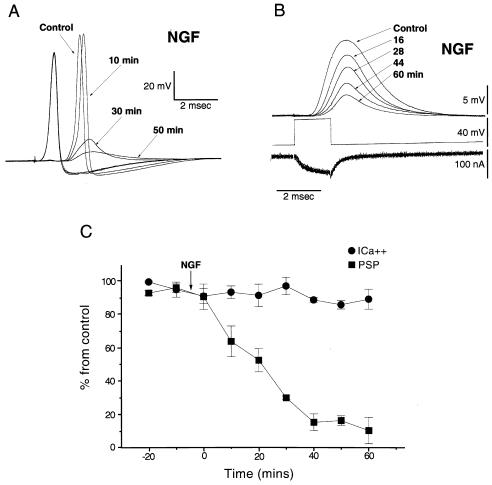
Figure 1.
NGF affects PSP but not presynaptic spike or calcium current. (A) Pre- and postsynaptic spikes from the squid giant synapse, before and after extracellular application of NGF (100 ng/ml). The postsynaptic response was markedly reduced 50 min after NGF application. No significant change in the presynaptic spike was observed. (B) Simultaneous recording of presynaptic calcium current (ICa2+) and PSP evoked by a depolarizing voltage-clamp step before (control) and after NGF superfusion (100 ng/ml). Note that PSP decreased in amplitude, whereas presynaptic ICa2+ did not change. (C) Normalized time course of the effect of 100 ng/ml of extracellular NGF on PSP (■) and ICa2+ (•) (1) using the same experimental protocol as in B (n = 4). Plot is the percentage of remaining PSP or ICa2+ at a given time; bars represent standard deviation.
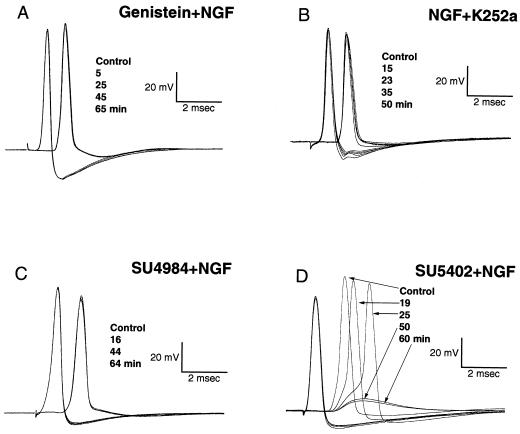
Figure 2.
NGF effect on synaptic transmission involves tyrosine phosphorylation. Pre- and postsynaptic potentials before and after extracellular application of 100 ng/ml of NGF. NGF was superfused after the postsynaptic terminal had been microinjected with 10 μM genistein (A) or cells had been preincubated for 10 min with 100 nM KT 252a. (B) NGF was applied at the same concentration as in A in cells preincubated for 10 min with 20 μM SU4984 (C) or 20 μM SU5402 (D). Shown are traces before and after NGF superfusion at the indicated times. Note that SU5402 did not block the NGF effect on synaptic transmission and that none of the tyrosine kinase inhibitors affected pre- or postsynaptic spike generation.
NGF Does Not Affect Presynaptic Calcium Current.
Presynaptic voltage clamp protocols were implemented to determine the effect of NGF on presynaptic calcium currents and on the associated postsynaptic response (19). Superfusion with an identical concentration of NGF, similar to those described above, produced comparable inhibition of the postsynaptic potential both in magnitude and time course (n = 4). However, no significant change in presynaptic calcium current (ICa2+) amplitude was observed (94% ± 8% of initial value) at 60 min after NGF superfusion, while the amplitude of the excitatory postsynaptic potential (EPSP) was markedly reduced (12.4% ± 6% of initial value) at 60 min (Fig. 1 B and C).
Reduction of PSP Amplitude by Tyrosine Kinase Receptor Activation is NGF-Specific.
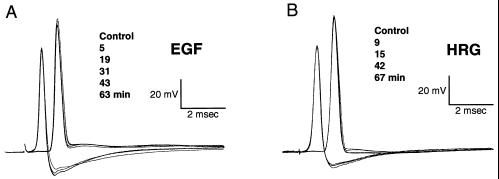
To determine whether PSP amplitude reduction is a general action of peptide growth factors, the effects of 50-min exposure to NGF 100 ng/ml on PSP were compared with those of BDNF (50 ng/ml to 300 ng/ml), NT3 (50 ng/ml to 300 ng/ml), EGF (50 ng/ml to 400 ng/ml), aFGF, (50 ng/ml to 400 ng/ml), and HRG (50 ng/ml to 400 ng/ml). Indeed, as illustrated in Fig. 3A and B, only NGF induced a significant reduction of PSP amplitude. The effects of the different growth factors were tested under current-clamp experiments (n = 4 for each of the five ligands) as well as in voltage-clamp mode (n = 4 for EGF and n = 2 for HRG). Calcium currents were not affected by any of the growth factors at the concentrations tested (89% ± 12% for EGF and an average of 92% for HRG from initial value at 60 min). This finding indicates that while in mammals there is promiscuity in terms of the neurotrophins–receptor interaction (1), in the squid system NGF is the only ligand that significantly activates this cascade of events.
Figure 3.
Specificity of NGF on PSP modulation. Pre- and postsynaptic potentials registered after superfusion of 300 ng/ml of EGF (A) and HRG (B). Identical results were obtained with NT3, BDNF, and aFGF (not shown).
Pharmacological Profile of the Squid NGF Tyrosine Kinase Receptor.
A potent and relatively selective inhibitor of Trk receptors, K252a (24, 25), was used to determine the nature of the NGF receptor. Bath application of 100 nM K252a (n = 4) 10 min before NGF (100 ng/ml) treatment produced a significant block of NGF effect on the EPSP without affecting any other aspect of synaptic transmission, as reflected by the stability of the pre- and postsynaptic spikes (Fig. 2B). Further analysis of this receptor was implemented by using a new class of protein tyrosine kinase inhibitors, 3-[4-(1-formylpiperazin-4yl)-benzylidenyl]-2-indolinone (SU4984) and 3-[(3-(2-carboxyethyl)-4-methylpyrrol-2-yl)methylenel-2-indolinone (SU5402) (26). Microinjection of SU4984 (20 μM) in the postsynaptic terminal as well as extracellular preincubation prevented the effect of NGF on synaptic transmission, while SU5402 at the same concentration did not interfere with the NGF action (n = 3 for the preincubation and n = 2 for the microinjection in both cases) (Fig. 2 C and D). Such results also provided further evidence concerning the postsynaptic site of action of NGF. These compounds display differential specificity toward various RTKs (26). SU5402 is a relatively selective inhibitor of FGRK1, with low activity against other RTKs, while SU4984 exhibits rather broad specificity.
Molecular Identification of the NGF-Activated Tyrosine Kinase Receptor in the Squid Giant Synapse, a TrkA-Like Protein.
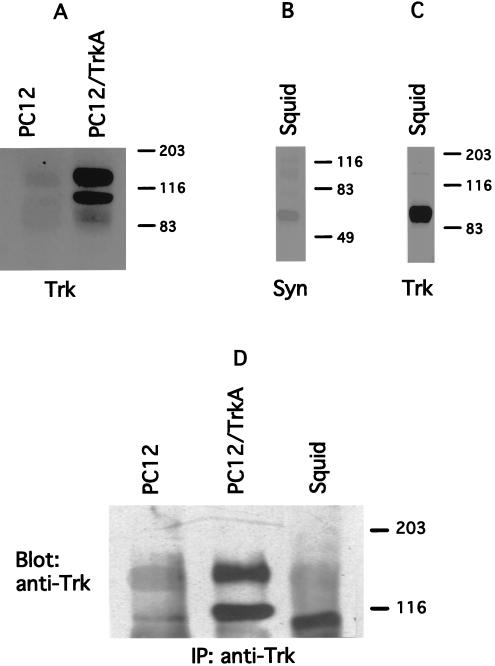
Because the ligand selectivity and pharmacology of synaptic modulation by NGF strongly suggested that the tyrosine kinase receptor is of the TrkA type, a series of experiments was designed to identify such a protein. Western blot analysis (n = 3) and immunoprecipitations (n = 3) were performed by using polyclonal antibodies specific for Trk receptors in mammals (see Methods). These blots revealed that the stellate ganglion, which houses the giant synapse (a preparation identical to that used for the electrophysiological experiments; see Materials and Methods) contains two proteins immunologically related to mammalian TrkA, a ≈140-kDa and a 95-kDa protein. The ≈95-kDa protein is more abundant than the prominent mammalian 140-kDa product (27) and is of lower molecular weight than the low molecular weight band (gp110) seen in transfected cells stably expressing TrkA (n = 3) or in parental pheochromocytoma-derived PC12 cells (n = 3) (Fig. 4 A, C, and D).
Figure 4.
Biochemical identification of TrkA-like receptors in squid nervous system. Parenteral PC12 cells (PC12) and PC12 cells overexpressing TrkA receptor (PC12/TrkA) were used as positive controls for anti-Trk antibodies. Antibodies against squid syntaxin C2A domain (see Materials and Methods) were used as positive control for the squid membrane preparation. (A–C) Western blot analysis of cell lysates from PC12, PC12/TrkA, and squid giant synapse. All cell extracts were blotted with anti-Trk antibodies (Trk) except B, which was blotted with antisyntaxin. (D) Lysates of the three preparations were immunoprecipitated with anti-Trk antibodies and were analyzed by immunoblotting with the same antibody.
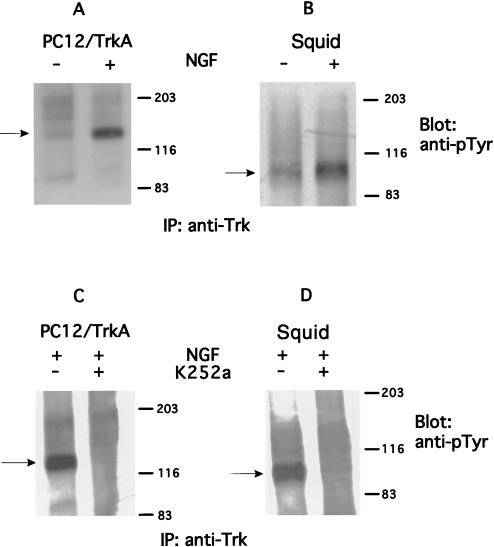
Because TrkA receptors undergo tyrosine autophosphorylation in response to NGF, further characterization of the proteins identified with the anti-Trk antibodies was undertaken. Stellate ganglia were incubated with NGF (200 ng/ml) for 15 min at 22°C in artificial sea water. Lysates of stimulated and unstimulated ganglia were subjected to immunoprecipitation with polyclonal antibodies against Trk, followed by immunoblotting with antibodies against phosphotyrosine (n = 2). Tyrosine phosphorylation of the putative squid TrkA receptor (the low molecular weight band) was found to be stimulated in response to NGF as shown in Fig. 5B. These immunoblots were compared with experiments run in parallel by using PC12 cells stably transfected with TrkA (PC12/TrkA) following the same protocol as that for squid giant synapse but at 37°C in regular DMEM (n = 2) (Fig. 5A). Note that, as expected, mammalian TrkA receptor was tyrosine phosphorylated on NGF exposure at the 140-kDa form, while the squid TrkA-like protein exhibited an apparent molecular weight of 95 kDa. Analysis of autophosphorylation of “squid TrkA” in synapses and PC12/TrkA cells (n = 2 for both) preincubated for 15 min with 200 nM K252a demonstrated that the inhibitor was able to decrease significantly the level of NGF stimulation of tyrosine phosphorylation on squid 95-kDa TrkA form as well as on the mammalian TrkA (gp140) (Fig. 5 C and D). These results corroborate the identity of the squid TrkA immunologically, pharmacologically, and biochemically and are consistent with the electrophysiological results demonstrating the blocking effect of TrkA activation on PSP amplitude.
Figure 5.
NGF treatment of PC12/TrkA and squid giant synapse promotes phosphorylation of TrkA receptors that can be blocked by the protein kinase inhibitor K252a. Lysates of NGF-stimulated (+) or NGF-unstimulated (−) PC12 cells (A) or squid giant synapse (B) were immunoprecipitated with anti-Trk antibodies (IP: anti-Trk) and were immunoblotted with antiphosphotyrosine antibodies (anti-pTyr). PC12 cells (C) and synapses (D) were preincubated for 15 min with 200 nM K252a (+) or not preincubated with K252a (−) then stimulated with NGF (+) and submitted to the same protocol as in A and B. The migration of the TrkA-phosphorylated product is indicated by the arrows. In both cases, NGF concentration was 200 ng/ml and was performed for 15 min.
DISCUSSION
Neurotrophins are important mediators of development, synaptic function, and cell survival in the vertebrate central nervous system and peripheral nervous system. Many actions of neurotrophic effects require de novo gene expression and protein synthesis. However, a series of studies in vertebrates has indicated acute synaptic neuromodulation by such growth factors, mostly at the presynaptic site, resulting in synaptic potentiation (reviewed in ref. 4). A few reports suggest growth factor-mediated postsynaptic facilitatory modulation; however, the pre- or postsynaptic site of action in most cases was evaluated only indirectly (28–30).
In the present report, an action of NGF on the squid giant synapse is described. Treatment of the synapse with NGF for a period of minutes reduces the amplitude of the synaptic potential without affecting the presynaptic spike or calcium current. This effect is produced only by NGF and not by other peptide growth factors tested. The site of action of squid NGF modulation is postsynaptic and is consistent with previous findings concerning modulation of synaptic transmission by tyrosine phosphorylation (18). The findings imply that the level of tyrosine phosphorylation mediated by NGF receptor activation can play a role in postsynaptic current modulation under physiological conditions.
NGF Signals Through TrkA in the Squid Giant Synapse.
Present experiments demonstrate that the NGF effect on PSP amplitude requires tyrosine kinase activity. This conclusion is based on the modulation block by both selective (K252a, SU4984) and nonselective (genistein) protein tyrosine kinase inhibitors. K252a is an indolocarbazole derived from Nocardiopsis sp. (31) that is known to inhibit TrkA by way of interactions at the tyrosine kinase domain, not by association with ligand, extracellular, or transmembrane domains (25). The pharmacological findings, particularly those of K252a, indicate that NGF acts through a TrkA-like receptor. Moreover, although mammals and mollusks are phylogenetically distant, antibodies raised against mammalian Trk receptors recognized a protein that was tyrosine phosphorylated in response to NGF stimulation in lysates prepared from squid nervous tissue.
Neurotrophins in Invertebrates: Identities and Signaling Pathway.
To date, only a small number of candidate growth factors and RTKs with specific role have been demonstrated in invertebrates (32–37). Moreover, identification of such ligands and receptors in invertebrates has occurred only recently (16, 17). The one neurotrophin and Trk receptor identified in an invertebrate (the freshwater snail Lymnea stagnalis) differs substantially from the receptor reported in this paper, as the snail cysteine-rich neurotrophin binds to a p75-like receptor and modulates calcium currents. Conversely, the neurotrophin receptor Ltrk binds human NT3, but not NGF. There are, nevertheless, some common features between these two molecules, as Ltrk is sequence-related to the vertebrate Trk receptors. Such result parallels our findings that antibodies against mammalian Trk receptors identified a protein that undergoes autophosphorylation on NGF exposure. Such autophosphorylation is prevented by K252a, as would be expected for TrkA-like receptors, despite having a different molecular weight than its mammalian counterpart (gp140), suggesting that the squid receptor is shorter than the vertebrate counterpart.
In vertebrates, TrkA is activated mainly by NGF, although NT3, NT4/5, NT6, and the recently identified NT7 can also bind TrkA, implying the existence, in vertebrates, of a number of NGF-like neurotrophins (2, 5, 38). In addition, some actions of NGF are mimicked by FGF in vertebrate systems (39). However, in the squid, these neurotrophins do not produce the TrkA receptor activation brought about by NGF.
From the present and previous findings (18), it is evident that the level of tyrosine phosphorylation may play a key role in modulation of synaptic transmission. The tyrosine kinase signaling in this system could be supported by TrkA itself or by one of the associated downstream tyrosine kinases (7). Although no information is available regarding the target of the tyrosine kinase(s), in this case the target is obviously located at the postsynaptic site and the peptide must act directly or indirectly to reduce the receptor sensitivity to the synaptic transmitter substance. Indeed, in vertebrates, Trk-mediated tyrosine phosphorylation modulates glutamate receptors of the N-methyl-d-aspartate (NMDA) type, both involving the pore and nonpore forming subunits of N-methyl-d-aspartate receptors (40, 41). In contrast, in the squid giant synapse, the postsynaptic response is mediated by an α-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid (AMPA)-like receptor (Fig. 6), but the molecular identification of this receptor is absent (42). Moreover, since some characteristics of invertebrate glutamate receptors, such as chloride conductance (43), are not retained by their vertebrate counterparts, it is probable that the modulation produced by NGF of squid giant synapse involves target(s) not yet identified in vertebrates.
Figure 6.
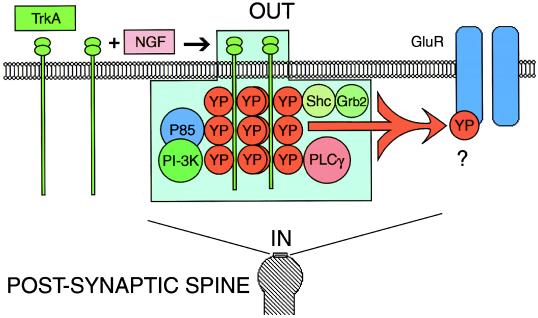
Induction of tyrosine phosphorylation by NGF modulates transmission at the postsynaptic site of squid giant synapse. Activation of TrkA-like receptors in the postsynaptic site by NGF produces autophosphorylation of the receptor cytoplasmic tyrosine residues (YP). Associated downstream signaling molecules to tyrosine phosphorylated squid TrkA are still unknown. Shown are some of the molecules known to bind tyrosine-phosphorylated TrkA: phosphatidylinositol 3 kinase (PI-3K), the adaptor protein P85 (P85), phospholipase Cγ (PLCγ), and Shc (Shc) in other systems. TrkA can signal directly or indirectly, resulting in tyrosine phosphorylation (YP) and inhibition of excitatory postsynaptic potential by direct or indirect inhibition of glutamate receptors (GluR).
In conclusion, electrophysiological, biochemical, and pharmacological data demonstrate modulation of transmission by NGF at the squid giant synapse. This modulation occurs by means of a cascade that includes a TrkA-like-mediated tyrosine phosphorylation and results in an inhibition of the postsynaptic response.
Acknowledgments
We thank David H. Lau for comments on the manuscript. R.L. received support from National Institutes of Health–National Institute for Neurological Disorders and Strokes (NINDS) (Grant NS13742) and J.S. received support from Sugen.
ABBREVIATIONS
- NGF
nerve growth factor
- BDNF
brain derived growth factor
- RTK
receptor tyrosine kinase
- EGF
epidermal growth factor
- aFGF
acidic fibroblast growth factor
- EPSP
excitatory postsynaptic potential
References
- 1.Chao M, Hempstead B. Trends Neurosci. 1995;18(7):321–325. [PubMed] [Google Scholar]
- 2.Lai K, Fu W, Ip F, Ip N. Mol Cell Neurosci. 1998;11:64–76. doi: 10.1006/mcne.1998.0666. [DOI] [PubMed] [Google Scholar]
- 3.Greene L, Kaplan D. Curr Opin Neurobiol. 1995;5:579–587. doi: 10.1016/0959-4388(95)80062-x. [DOI] [PubMed] [Google Scholar]
- 4.Berninger B, Poo M. Curr Opin Neurobiol. 1996;6:324–330. doi: 10.1016/s0959-4388(96)80115-2. [DOI] [PubMed] [Google Scholar]
- 5.McDonald N, Chao M. J Biol Chem. 1995;270(34):19669–19672. doi: 10.1074/jbc.270.34.19669. [DOI] [PubMed] [Google Scholar]
- 6.Jing S, Tapley P, Barbacid M. Neuron. 1992;9:1067–1079. doi: 10.1016/0896-6273(92)90066-m. [DOI] [PubMed] [Google Scholar]
- 7.Schlessinger J, Ulrich A M. Neuron. 1992;9:383–391. doi: 10.1016/0896-6273(92)90177-f. [DOI] [PubMed] [Google Scholar]
- 8.Kaplan D, Miller F. Curr Opin Cell Biol. 1997;9:213–221. doi: 10.1016/s0955-0674(97)80065-8. [DOI] [PubMed] [Google Scholar]
- 9.Riccio A, Pierchala B, Ciarallo C, Ginty D. Science. 1997;277:1097–1100. doi: 10.1126/science.277.5329.1097. [DOI] [PubMed] [Google Scholar]
- 10.Lewin G, Barde Y. Annu Rev Neurosci. 1996;19:289–317. doi: 10.1146/annurev.ne.19.030196.001445. [DOI] [PubMed] [Google Scholar]
- 11.Barde Y. J Neurobiol. 1994;25:1386–1403. doi: 10.1002/neu.480251102. [DOI] [PubMed] [Google Scholar]
- 12.Ridgway R, Syed N, Lukowiak K, Bulloch A. J Neurobiol. 1991;22:377–390. doi: 10.1002/neu.480220406. [DOI] [PubMed] [Google Scholar]
- 13.Syed N, Richardson P, Bulloch A. J Neurobiol. 1996;29:293–303. doi: 10.1002/(SICI)1097-4695(199603)29:3<293::AID-NEU2>3.0.CO;2-4. [DOI] [PubMed] [Google Scholar]
- 14.Magoski N, Bulloch A. Soc Neurosci Abst. 1996;22:754. [Google Scholar]
- 15.Wildering W, Lodder J, Kits K, Bulloch A. J Neurophysiol. 1995;74:2778–2781. doi: 10.1152/jn.1995.74.6.2778. [DOI] [PubMed] [Google Scholar]
- 16.Fainzilber M, Smit A, Syed N, Wildering W, Hermann P, van der Schors R, Jimenez C, Li K, Minnen J, Bulloch A, et al. Science. 1996;274:1540–1543. doi: 10.1126/science.274.5292.1540. [DOI] [PubMed] [Google Scholar]
- 17.Kesteren R, Fainzilber M, Hauser G, Minnen J, Vreugdenhil E, Smit A, Ibanez C, Geraerts P, Bulloch A. EMBO J. 1998;17(9):2534–2542. doi: 10.1093/emboj/17.9.2534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Llinás R, Moreno H, Sugimori M, Mohammadi M, Schlessinger J. Proc Natl Acad Sci USA. 1997;94:1990–1994. doi: 10.1073/pnas.94.5.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Llinás R, Steinberg I, Walton K. Biophys J. 1981;33(3):289–321. doi: 10.1016/S0006-3495(81)84898-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Dikic I, Batzer A G, Blaikie P, Obermeier A, Ullrich A, Schlessinger J, Margolis B. J Biol Chem. 1995;270(25):15125–15129. doi: 10.1074/jbc.270.25.15125. [DOI] [PubMed] [Google Scholar]
- 21.Kouhara H, Hadari Y R, Spivak-Kroizman T, Schilling J, Bar-Sagi D, Lax I, Schlessinger J. Cell. 1997;30:89:693–702. doi: 10.1016/s0092-8674(00)80252-4. [DOI] [PubMed] [Google Scholar]
- 22.Fukuda M, Moreira J E, Lewis F M, Sugimori M, Niinobe M, Mikoshiba K, Llinás R. Proc Natl Acad Sci USA. 1995;92(23):10708–10712. doi: 10.1073/pnas.92.23.10708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Dikic I, Schlessinger J, Lax I. Curr Biol. 1994;4(8):702–708. doi: 10.1016/s0960-9822(00)00155-x. [DOI] [PubMed] [Google Scholar]
- 24.Knight E, Connors T, Maroney A, Angeles T, Hudkins R, Dionne C. Anal Biochem. 1997;247:376–381. doi: 10.1006/abio.1997.2093. [DOI] [PubMed] [Google Scholar]
- 25.Angeles T, Yang S, Steffler C, Dionne C. Arch Biochem Biophys. 1998;349(2)15:267–274. doi: 10.1006/abbi.1997.0490. [DOI] [PubMed] [Google Scholar]
- 26.Mohammadi M, McMahon G, Sun L, Tang C, Hirth P, Yeh B, Hubbard S, Schlessinger J. Science. 1997;276:955–960. doi: 10.1126/science.276.5314.955. [DOI] [PubMed] [Google Scholar]
- 27.Martin-Zanca D, Oskam R, Mitra G, Copeland T, Barbacid M. Cell Biol. 1989;9(1):24–33. doi: 10.1128/mcb.9.1.24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Li Y, Xu Y, Ju D, Lester D, Davidson N, Schuman E. Proc Natl Acad Sci USA. 1998;95:10884–10889. doi: 10.1073/pnas.95.18.10884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Wang X, Poo M. Neuron. 1997;19:825–835. doi: 10.1016/s0896-6273(00)80964-2. [DOI] [PubMed] [Google Scholar]
- 30.Lockhart S, Turrigiano G, Birren S. J Neurosci. 1997;17(24):9573–9582. doi: 10.1523/JNEUROSCI.17-24-09573.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kase H, Iwashashi K, Matsuda Y. J Antibiot. 1986;39:1059–1065. doi: 10.7164/antibiotics.39.1059. [DOI] [PubMed] [Google Scholar]
- 32.Smit A, Vreudenhil E, Ebberink R, Geraerts W, Klootwijk J, Joosse J. Nature (London) 1988;331:535–538. doi: 10.1038/331535a0. [DOI] [PubMed] [Google Scholar]
- 33.Roovers E, Vincent M, Kesteren V, Geraerts W, Planta R, Vreugdenhil E, Heerikhuizen H. Gene. 1995;162:181–188. doi: 10.1016/0378-1119(95)00323-x. [DOI] [PubMed] [Google Scholar]
- 34.Jonas E, Knox R, Kaczmarek L, Schwartz J, Solomon D. J Neurosci. 1996;16:1645–1658. doi: 10.1523/JNEUROSCI.16-05-01645.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Pulido D, Campuzano S, Koda T, Modolell J, Barbacid M. EMBO J. 1992;11:391–404. doi: 10.1002/j.1460-2075.1992.tb05067.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Wilson C, Goberdhan D, Steller H. Proc Natl Acad Sci USA. 1993;90:7109–7113. doi: 10.1073/pnas.90.15.7109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Oishi I, Sugiyama S, Liu Z, Yamamura H, Nishida Y, Minami Y. J Biol Chem. 1997;272:11916–11923. doi: 10.1074/jbc.272.18.11916. [DOI] [PubMed] [Google Scholar]
- 38.Barker P A, Lomen-Hoerth C, Gensch E M, Meakin S O, Glass D J, Shooter E M. J Biol Chem. 1993;268(20):15150–15157. [PubMed] [Google Scholar]
- 39.Rydel E, Greene L. J Neurosci. 1987;7(11):3639–3653. doi: 10.1523/JNEUROSCI.07-11-03639.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Suen P, Wu K, Levine E, Mount H, Xu J, Lin S, Black I. Proc Natl Acad Sci USA. 1997;94(15):8191–8195. doi: 10.1073/pnas.94.15.8191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Lin S, Wu K, Levine E, Mount H, Suen P, Black I. Brain Res Mol Brain Res. 1998;55(1):20–27. doi: 10.1016/s0169-328x(97)00349-5. [DOI] [PubMed] [Google Scholar]
- 42.De Santis A, Messenger J B. Q J Exp Physiol. 1989;74(2):219–222. doi: 10.1113/expphysiol.1989.sp003259. [DOI] [PubMed] [Google Scholar]
- 43.Cleland T. Mol Neurobiol. 1996;13(2):97–136. doi: 10.1007/BF02740637. [DOI] [PubMed] [Google Scholar]