Abstract
Evidence that lesions of the basolateral amygdala complex (BLC) impair memory for fear conditioning in rats, measured by lack of “freezing” behavior in the presence of cues previously paired with footshocks, has suggested that the BLC may be a critical locus for the memory of fear conditioning. However, evidence that BLC lesions may impair unlearned as well as conditioned freezing makes it difficult to interpret the findings of studies assessing conditioned fear with freezing. The present study investigated whether such lesions prevent the expression of several measures of memory for contextual fear conditioning in addition to freezing. On day 1, rats with sham lesions or BLC lesions explored a Y maze. The BLC-lesioned rats (BLC rats) displayed a greater exploratory activity. On day 2, each of the rats was placed in the “shock” arm of the maze, and all of the sham and half of the BLC rats received footshocks. A 24-hr retention test assessed the freezing, time spent per arm, entries per arm, and initial entry into the shock arm. As previously reported, shocked BLC rats displayed little freezing. However, the other measures indicated that the shocked BLC rats remembered the fear conditioning. They entered less readily and less often and spent less time in the shock arm than did the control nonshocked BLC rats. Compared with the sham rats, the shocked BLC rats entered more quickly and more often and spent more time in the shock arm. These findings indicate that an intact BLC is not essential for the formation and expression of long-term cognitive/explicit memory of contextual fear conditioning.
Keywords: avoidance/freezing/excitotoxic lesions/classical conditioning
Studies from many laboratories have reported that in rats, lesions or inactivation of the basolateral amygdala complex (BLC; the set of lateral, basal, and accessory basal nuclei of the amygdala) decrease the immobility or “freezing” (defined as lack of movement except for respiration) displayed in the presence of specific cues or contexts previously paired with footshock (1–7). Such findings have suggested that the BLC may be critical for storing cue–footshock or context–footshock associations and that the BLC may be a locus of the neuroplasticity that mediates fear conditioning (2, 5). A major difficulty with these interpretations is that lesions and other treatments that disrupt amygdala functioning also increase locomotor activity (8–10) and decrease unlearned fear, or anxiety (11–15). To draw conclusions about the effects of brain lesions on learning and memory, it is essential to know that the lesions by themselves do not affect the behavior used to make inferences about learning and memory. Thus, to date, the findings of experiments in which freezing is used as the index of learning have not provided unequivocal evidence implicating the BLC as a critical region of the brain for fear-conditioning memory. To investigate further the involvement of the BLC in memory for contextual fear-conditioning, the present experiment used retention testing procedures that included several response measures in addition to freezing behavior.
MATERIALS AND METHODS
Subjects and Surgical Procedures.
The subjects were 57 adult male Sprague–Dawley rats (Charles River Breeding Laboratories) weighing between 275 and 350 g at the time of surgery. They were individually housed in a temperature-controlled (22°C) room and maintained on a standard 12-hr light/12-hr dark cycle with food and water freely available. One week after their arrival, the rats were anesthetized with nembutal (55 mg/kg) and supplemented with 0.2 ml of atropine sulfate to prevent obstructed breathing. Twenty-four rats were given stereotaxically guided bilateral excitotoxic lesions of the BLC with N-methyl-d-aspartic acid: one infusion of 0.2 μl at 2.6 mm posterior to bregma, 5.0 mm lateral to bregma, and 7.8 mm ventral from the skull surface; and a second infusion of 0.1 μl at 0.3 mm dorsal to the first infusion site. Coordinates were based on the Swanson rat-brain atlas (16). The neurotoxin was infused via a 30-gauge cannula at the rate of 0.5 μl/min controlled by a micropump (Sage Instruments, Boston) and timer and was allowed to diffuse for 3 min. After the surgery, the rats recovered in an incubator under close supervision. Sham-lesioned rats (n = 8) underwent the same procedure but did not receive infusions.
Behavioral Procedures.
One week after the surgery, the rats received contextual fear-conditioning training. On day 1 (habituation day), the rats were allowed to explore a Y maze for 8 min. The three arms, separated by 120°, were of the same length (0.5 m) and depth (0.18 m), but were differently shaped, colored, and textured. The arms were covered with translucent Plexiglas lids. Total time spent freezing and latency to each arm entry were recorded. On day 2 (training day), the rats were placed in one arm (shock arm) that was blocked off from the rest of the maze. After 120 sec, some rats received the first of four footshocks (1 mA ac, 1 sec) delivered at 1-min intervals through stainless steel floor plates. One minute after the last footshock, the rats were returned to their home cages. The total time freezing after each footshock was recorded. Other rats were placed in the shock arm for the same period of time but received no footshock. On day 3 (test day), the rats were placed in an arm where they did not receive footshock and were allowed access to all arms of the maze for 8 min. Total time spent freezing and latency to each arm entry were recorded. No shocks were delivered during the test. The subjects’ behavior was observed via a mirror suspended 1.2 m above the Y maze, thus excluding the experimenter as an extra maze cue. Before and after each session, the subjects were retained in a room adjacent to the experiment room for 1 hr.
Nociception.
A subgroup of the BLC (n = 6) and sham-lesioned (n = 3) rats were tested for footshock sensitivity in a Plexiglas box (0.23 × 0.21 m) with a metal grid floor connected to a multisetting custom-made shock generator, which delivered scrambled shocks (0.1–0.56 mA). Two responses were measured: flinch, defined as the retraction of at least one paw, and jump, the retraction of all four paws.
Drugs.
The nembutal sodium anesthetic solution (Abbott) was injected i.p. at 55 mg/kg. The atropine sulfate (Phoenix Pharmaceuticals, St. Joseph, MO) was also injected i.p. The N-methyl-d-aspartic acid (Sigma) was dissolved in saline to a final concentration of 20 μg/ml immediately before the surgeries and was kept on ice in a tightly sealed, lightproof vial.
Histology.
At the end of the behavioral testing, all rats were deeply anesthetized with an overdose of sodium pentobarbital (200 mg/kg, i.p.; Sigma) and perfused intracardially with 0.9% saline solution followed by 10% formalin. The brains were removed and stored in 10% formalin overnight, then transferred to 30% sucrose in 10% formalin solution. Forty-micron sections were cut on a freezing microtome, mounted on gelatin-coated slides, and stained with cresyl violet. The slides were later examined under a light microscope by two independent observers. The extent of the lesions was drawn on schematic representations of the rat forebrain (17).
Statistical Analysis.
Initial latency to enter the shock arm, total time per arm, total number of entries, and time spent per entry into the shock arm were calculated from the latency to each arm entry measure. A repeated-measures ANOVA assessed the effects of the lesion and footshock treatments on the freezing during training. ANOVAs were used to determine the treatment effect for the time per arm, freezing, number of entries, first entry into the shock arm, and footshock response measures. Fisher’s tests were used for all post hoc comparisons. Analysis with a paired t test examined the differences between pre- and posttraining levels of dependent variables for each treatment condition. Probabilities smaller than 0.05 were considered significant.
RESULTS
Histology.
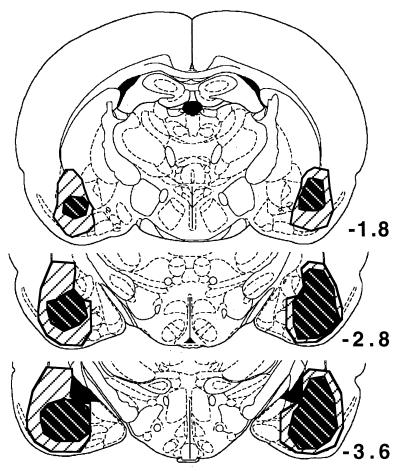
Fig. 1 illustrates the extent of the BLC lesions (n = 21), which in some cases included, in addition to the BLC, the endopiriform nucleus, ventral portions of the caudate nucleus, part of the piriform cortex, and occasional damage to the corticomedial amygdala. The lesions did not involve the central nucleus of the amygdala. Three animals with primarily unilateral lesions of the BLC were excluded from the behavioral analysis.
Figure 1.
Extent of the smallest (black-hatched) and largest (white-hatched) BLC lesions. Numbers indicate the relative position of the coronal sections (in millimeters) posterior to Bregma (17). [Adapted from ref. 17 with permission from Academic Press, Orlando, FL.
Habituation.
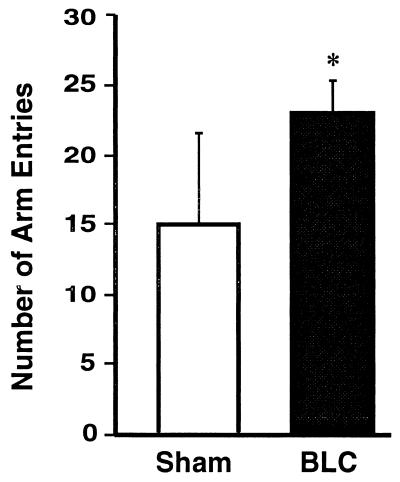
On day 1, all of the rats explored all three arms of the maze and displayed no freezing. However, the BLC-lesioned rats entered the maze arms more often than did sham-lesioned rats [F1,27 = 10.92, P < 0.005, Fig. 2].
Figure 2.
Total number of arm entries during the habituation period, day 1, for sham-lesioned rats (Sham, open bar) and for rats with BLC lesions (BLC, black bar). Bars represent means (± SEM). ∗, P < 0.005.
Training.
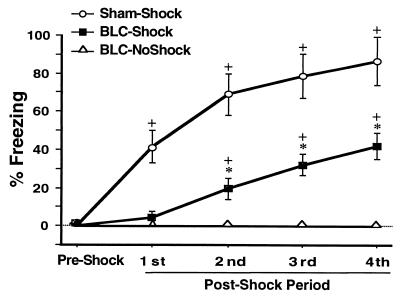
On day 2, all of the sham-lesioned (sham) and half of the BLC-lesioned rats (shocked-BLC, n = 10) received footshocks while restricted to the shock arm of the maze. The other BLC-lesioned rats (nonshocked-BLC, n = 11) were also placed in the shock arm but did not receive any footshocks. Fig. 3 shows that the series of footshocks resulted in increased freezing [F3,48 = 36.41, P < 0.0001]. The training effect was the result of increased freezing in both the sham and the shocked-BLC groups as indicated by a lack of a lesion × training interaction [F3,48 = 0.77, not significant (ns)]. Further analysis with a paired t test confirmed that both the sham and the shocked-BLC rats spent significantly more time freezing after the last footshock compared with the preshock period (P < 0.005 for both). In the presence of a significant effect of treatment [F2,26 = 39.38, P < 0.0001], post hoc tests revealed that the shocked-BLC rats spent less time freezing than the sham rats, but more time freezing than the nonshocked-BLC rats (P < 0.005 for both comparisons). Comparison of freezing in shocked-BLC rats with partial lesions with shocked-BLC rats with complete lesions revealed that lesion size did not account for the training-induced increase in freezing [F1,8 = 3.68, ns; also, training × lesion size interaction, ns].
Figure 3.
Mean percentage of time spent freezing (± SEM) on day 2 before (preshock) and during (1–4 postshock periods) training in sham rats that received footshocks (○), BLC-lesioned rats that received footshocks (▪), and BLC-lesioned rats that did not receive footshocks (▵). ∗, P < 0.005 compared with Sham-Shock and BLC-NoShock; +, P < 0.005 compared with the preshock period.
Retention Testing.
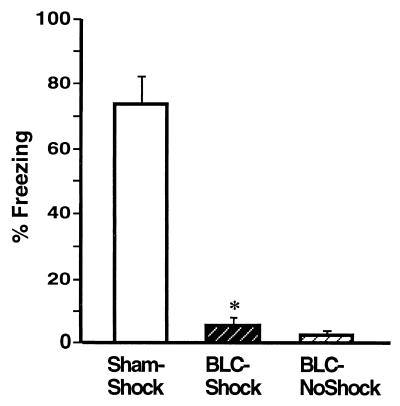
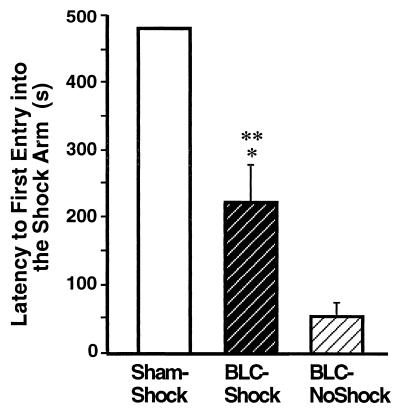
Fig. 4 shows the freezing performance of animals in each group on the 8-min retention test on day 3. In agreement with previous reports (1, 2), the BLC lesions significantly reduced freezing [F2,26 = 82.96, P < 0.0001]. Post hoc tests confirmed that shocked-BLC rats showed less freezing than did sham rats (P < 0.0001) but did not differ significantly from the nonshocked-BLC rat controls. The deficit in freezing did not depend on the extent of the lesions, as shocked-BLC rats with complete lesions did not differ from shocked-BLC rats with partial lesions [F1,8 = 0.01, ns]. Fig. 5 shows the shock arm entrance latencies of the three groups on the day 3 retention test. A main effect of treatment [F2,26 = 29.38, P < 0.001] and post hoc tests revealed that the entrance latencies of the shocked-BLC rats were shorter than those of the sham rats but longer than those of the nonshocked-BLC rats (P < 0.01 for both comparisons).
Figure 4.
Mean time spent freezing (± SEM) during the retention test on day 3 in Sham rats (Sham-Shock), BLC-lesioned rats that received footshocks (BLC-Shock), and BLC-lesioned rats that did not receive footshocks (BLC-NoShock) during the training on day 2. ∗, P < 0.0001 compared with the Sham-Shock group.
Figure 5.
Mean latencies, in seconds, to first entry into the shock arm (± SEM) during the retention test on day 3 for sham rats (Sham-Shock), BLC-lesioned rats that received footshocks (BLC-Shock), and BLC-lesioned rats that did not receive footshocks (BLC-NoShock) during the training on day 2. ∗, P < 0.01 compared with the Sham-Shock group; ∗∗, P < 0.01 compared with the BLC-NoShock group.
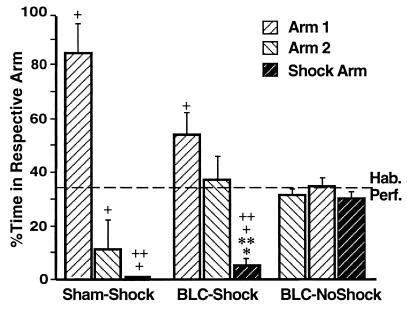
Fig. 6 shows the percentage of time spent in each maze arm on the day 3 retention test (bars) compared with that on the day 1 habituation (dashed line). The nonshocked-BLC rats spent comparable amounts of time in all of the arms on the retention test [F2,30 = 1.34, ns; all post hoc comparisons, ns]. In contrast, both the sham and the shocked-BLC animals spent different amounts of time in the three arms [F2,21 = 27.83, P < 0.0001 for the sham group; F2,27 = 12.71, P < 0.0001 for the shocked-BLC group]. Both groups spent less time in the shock arm than in arm 1, and in the case of the shocked-BLC rats, less time in the shock arm than in arm 2 (P < 0.005 for all comparisons). Furthermore, the sham rats, as well as the shocked-BLC rats, spent less time in the shock arm on day 3 than on day 1 (t = 4.11, P < 0.005 for the sham group; t = 6.91, P < 0.0001 for the shocked-BLC group). This selective avoidance of the shock arm is attributable to the context-footshock pairing, as the nonshocked-BLC rats, which underwent the same general behavioral procedures but did not receive footshocks, spent comparable amounts of time in the shock arm on day 1 and day 3 (t = 0.86, ns).
Figure 6.
Mean percentage of time spent per arm during the habituation period on day 1 (dashed line) and the retention test on day 3 (bars, ± SEM) for the three groups. ∗, P < 0.05 compared with the Sham-Shock group; ∗∗, P < 0.001 compared with the BLC-NoShock group; +, P < 0.005 compared with the habituation period; ++, P < 0.005 compared with the respective group’s percentage of time in arm 1.
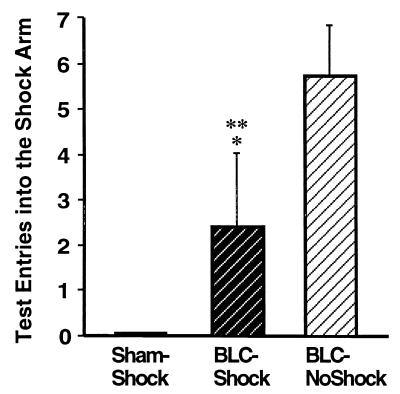
The number of entries into the shock arm made by the sham and each of the BLC-lesioned groups is shown in Fig. 7. The three groups differed in the number of shock arm entries [F2,84 = 65.07, P < 0.0001]. Post hoc comparisons showed that the number of entries of the shocked-BLC rats was greater than that of the sham controls but less than that of the nonshocked-BLC rats (P < 0.0001 for both). Although the shocked-BLC rats entered the shock arm, they quickly escaped. The average time per entry in the shock arm for the shocked-BLC rats was significantly less than that of their nonshocked-BLC controls (mean = 11.79 s, SE = 2.12 for the former, and mean = 26.45 s, SE = 2.09 for the latter; F1,19 = 33.98, P < 0.0001, data not shown).
Figure 7.
Mean number of entries into the shock arm (± SEM) during the retention test for the three groups. ∗, P < 0.0001 compared with the Sham-Shock group; ∗∗, P < 0.0001 compared with the BLC-NoShock group.
Nociception.
The sham and the BLC-lesioned rats did not differ in footshock sensitivity [F1,7 = 1.20, (ns) for flinch; F1,7 = 0.001, ns for jump].
DISCUSSION
The present experiment examined the effects of bilateral neurotoxic lesions of the BLC induced before training on the formation of memory for contextual fear conditioning. The rats were first allowed to explore an alley maze with three highly distinctive arms. The next day, all of the Sham and half of the BLC-lesioned rats received footshock in one arm of the maze. The BLC lesions attenuated but did not block the acquisition of fear conditioning, as indicated by increases in freezing during the fear conditioning training session. The BLC lesion effect on acquisition of fear conditioning was not caused by a lesion-induced decrease in nociception. Consistent with previous findings (1–4), the BLC lesions blocked freezing behavior assessed on the 24-hr retention test. However, the BLC lesions attenuated, but did not block, several other responses providing indices of memory for the context–footshock pairing. In comparison with the nonshocked-BLC controls, the shocked-BLC rats had longer latencies to enter the shock arm in the retention test, spent less time in the shock arm, and entered that arm less frequently. These findings provide strong evidence that the BLC lesions did not block the formation or expression of memory for the context-footshock training. The failure of BLC lesions to block fear-based memory, as indicated by avoidance of the shock arm, was not due to incomplete BLC lesions; the performance of rats with partial lesions was no better than that of animals with complete lesions.
It has been difficult to draw unambiguous conclusions concerning the effects of amygdala lesions on memory for contextual fear conditioning in studies using freezing as the measure of fear because such lesions also disrupt behavioral expression of unlearned fear (8, 10, 15). Thus, evidence that BLC lesions block freezing behavior assessed 24 hr after rats receive footshocks in a specific context, as found in this and previous studies (1–4), indicates only that the animals are unable to express memory for the training by freezing. Without controls for the effects of lesions on unconditioned freezing, such findings do not allow the conclusions that the lack of freezing indicates a selective blocking of learned fear. Interestingly, in the present study, the BLC lesions attenuated but did not block freezing on the fear conditioning training session, but completely blocked freezing on the retention test. The critical issue in interpreting these findings is the use of freezing as a measure of fear. If freezing expresses the emotional state of fear, then it would appear that an intact BLC is not critical for elicitation of fear during contextual fear conditioning training but is critical for expressing fear as assessed later on the retention test. Such findings seem difficult to reconcile with a general hypothesis that the BLC is a critical neural locus for contextual fear conditioning (2, 5).
Other findings of the present study clearly indicate that the BLC lesions did not prevent the rats from acquiring or expressing memory for the context–footshock pairing. The evidence that the BLC-lesioned rats avoided the arm where they had received footshocks suggests that the animals retained cognitive memory (18, 19) for the aversive training. It is important to note that the rats’ memory expressed strong valence; that is, the BLC-lesioned animals, like the sham controls, did not simply remember the place where they received the context-footshock fear conditioning, they avoided it. Clearly, an intact BLC is not critical for such fear-based memory. It is highly unlikely that the spared memory in BLC-lesioned rats was caused by acquisition of an instrumental response or “habit” during the training, as the footshock administered during the context conditioning was not contingent on any behavioral response.
Although the BLC lesions did not block the rats’ avoidance of the shock arm on the retention test, the avoidance responses were attenuated in comparison with those of the sham group (Figs. 4–7). The sham and BLC-lesioned groups differed significantly in all of the retention measures. However, it should also be noted that in comparison with the sham rats, the BLC-lesioned rats had greater locomotor activity on the habituation trial before the fear conditioning. Thus, lesion-induced increases in locomotor activity might well have influenced all of the retention test measures. That is, increases in locomotor activity would result in decreased freezing, shorter latencies to enter, and more entries into the shock arm, and as a consequence, an increase in the time spent in that arm. As the BLC lesions completely blocked freezing, it seems unlikely that that effect was due solely to an increase in locomotor activity. However, lesion-induced increases in locomotor activity might have attenuated freezing behavior and contributed significantly to the attenuating effects of the BLC lesions on the other behavioral measures of retention.
Our finding of preserved memory for fear-based learning in BLC-lesioned rats is consistent with those of several previous studies that used other types of aversive training. Selden et al. (20) reported that rats with BLC lesions tend to avoid an environment in which a tone was paired with a footshock. Parent et al. (21) found that BLC lesions induced after different amounts of training on a footshock-escape task did not block memory of the training. Degree of retention (as assessed by inhibitory avoidance) varied directly with the amount of original training in both control and BLC-lesioned rats. Additionally, several studies have reported that BLC lesions do not block either the acquisition or the retention of fear-based inhibitory avoidance training (22–24).
The present findings are thus consistent with other evidence indicating that an intact BLC is not critical for the formation of cognitive/explicit memory of fear-based learning. There is, however, extensive evidence that the BLC is critically involved in mediating neuromodulatory influences on the consolidation of long-term memory (25, 26). That is, although the BLC is not critical for acquisition or retention, it is critical for enabling the modulation of memory storage. Lesions of the BLC block the memory-modulating effects of posttraining systemic administration of drugs (24, 26, 27). Other findings indicate that posttraining microinfusions of drugs and hormones selectively into the BLC influence long-term memory storage (28–30) and that such effects are the result of modulation of consolidation of long-term explicit/declarative memory in other brain regions (25, 26). Such findings are consistent with evidence that the BLC modulates neuroplasticity in the hippocampus (31, 32) and hippocampally based memory (33) as well as evidence that the hippocampus is critically involved in the consolidation of declarative memory (34).
In summary, although BLC lesions blocked freezing behavior assessed 24 hr after contextual fear conditioning, the lesions attenuated, but did not block, memory of the fear conditioning as shown by behavioral measures that assess explicit memory. These findings thus strongly indicate that an intact BLC is not required for the acquisition and retention of memory of contextual fear conditioning.
Acknowledgments
We thank Binh Troung and Hilda Avila for excellent technical assistance. We also thank Dr. Larry Squire and Dr. Richard Thompson for helpful comments on a draft of this paper. This research was supported by University of California, Irvine Regent’s Fellowship (A.V.) and U.S. Public Health Service Grant MH12526 from the National Institute of Mental Health (J.L.M.).
ABBREVIATIONS
- BLC
basolateral complex of the amygdala
- ns
not significant
References
- 1.Maren S. J Neurosci. 1998;18:3088–3097. doi: 10.1523/JNEUROSCI.18-08-03088.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Maren S, Aharonov G, Fanselow M S. Behav Neurosci. 1996;110:718–726. doi: 10.1037//0735-7044.110.4.718. [DOI] [PubMed] [Google Scholar]
- 3.Kim J J, Rison R A, Fanselow M S. Behav Neurosci. 1993;107:1093–1098. doi: 10.1037//0735-7044.107.6.1093. [DOI] [PubMed] [Google Scholar]
- 4.Phillips R G, LeDoux J E. Behav Neurosci. 1992;106:274–285. doi: 10.1037//0735-7044.106.2.274. [DOI] [PubMed] [Google Scholar]
- 5.LeDoux J E, Muller J. Philos Trans R Soc London B. 1997;352:1719–1726. doi: 10.1098/rstb.1997.0154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Muller J, Corodimas K P, Fridel Z, LeDoux J E. Behav Neurosci. 1997;111:683–691. doi: 10.1037//0735-7044.111.4.683. [DOI] [PubMed] [Google Scholar]
- 7.Helmstetter F J, Bellgowan P S. Behav Neurosci. 1994;108:1005–1009. doi: 10.1037//0735-7044.108.5.1005. [DOI] [PubMed] [Google Scholar]
- 8.Blanchard D C, Blanchard R J. J Comp Physiol Psychol. 1972;81:281–290. doi: 10.1037/h0033521. [DOI] [PubMed] [Google Scholar]
- 9.Lorenzini C, Bucherelli C, Giachetti A, Mugnai L, Tassoni G. Physiol Behav. 1991;49:765–770. doi: 10.1016/0031-9384(91)90316-g. [DOI] [PubMed] [Google Scholar]
- 10.Burns L H, Annett L, Kelley A E, Everitt B J, Robbins T W. Behav Neurosci. 1996;110:60–73. doi: 10.1037//0735-7044.110.1.60. [DOI] [PubMed] [Google Scholar]
- 11.Walker D L, Davis M. J Neurosci. 1997;17:9375–9383. doi: 10.1523/JNEUROSCI.17-23-09375.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Mesches M H, Bianchin M, McGaugh J L. Neurobiol Learn Mem. 1996;66:324–340. doi: 10.1006/nlme.1996.0073. [DOI] [PubMed] [Google Scholar]
- 13.Sanders S K, Shekhar A. Pharmacol Biochem Behav. 1995;52:701–706. doi: 10.1016/0091-3057(95)00153-n. [DOI] [PubMed] [Google Scholar]
- 14.Bellgowan P S, Helmstetter F J. Behav Neurosci. 1996;110:727–736. doi: 10.1037//0735-7044.110.4.727. [DOI] [PubMed] [Google Scholar]
- 15.Kemble E D, Blanchard D C, Blanchard R J. Physiol Behav. 1990;48:1–5. doi: 10.1016/0031-9384(90)90251-x. [DOI] [PubMed] [Google Scholar]
- 16.Swanson L W. Brain Maps: Structure of the Rat Brain. Amsterdam: Elsevier; 1992. [Google Scholar]
- 17.Paxinos G, Watson C. The Rat Brain in Stereotaxic Coordinates. San Diego: Academic; 1997. [DOI] [PubMed] [Google Scholar]
- 18.Tolman E C. Purposive Behavior in Animals and Men. New York: Appleton-Century; 1932. [Google Scholar]
- 19.Tolman E C. Psychol Rev. 1948;55:189–208. doi: 10.1037/h0061626. [DOI] [PubMed] [Google Scholar]
- 20.Selden N R W, Everitt B J, Jarrard L E, Robbins T W. Neuroscience. 1991;42:335–350. doi: 10.1016/0306-4522(91)90379-3. [DOI] [PubMed] [Google Scholar]
- 21.Parent M B, Avila E, McGaugh J L. Brain Res. 1995;676:235–244. doi: 10.1016/0006-8993(95)00095-8. [DOI] [PubMed] [Google Scholar]
- 22.Parent M B, Quirarte G L, Cahill L, McGaugh J L. Behav Neurosci. 1995;109:803–807. doi: 10.1037//0735-7044.109.4.803. [DOI] [PubMed] [Google Scholar]
- 23.Roozendaal B, McGaugh J L. Neurobiol Learn Mem. 1996;65:1–8. doi: 10.1006/nlme.1996.0001. [DOI] [PubMed] [Google Scholar]
- 24.Tomaz C, Dickinson-Anson H, McGaugh J L. Proc Natl Acad Sci USA. 1992;89:3615–3619. doi: 10.1073/pnas.89.8.3615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Cahill L, McGaugh J L. Trends Neurosci. 1998;21:294–299. doi: 10.1016/s0166-2236(97)01214-9. [DOI] [PubMed] [Google Scholar]
- 26.McGaugh J L, Cahill L, Roozendaal B. Proc Natl Acad Sci USA. 1996;93:13508–13514. doi: 10.1073/pnas.93.24.13508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Roozendaal B, Portillo-Marquez G, McGaugh J L. Behav Neurosci. 1996;110:1074–1083. doi: 10.1037//0735-7044.110.5.1074. [DOI] [PubMed] [Google Scholar]
- 28.Quirarte G L, Roozendaal B, McGaugh J L. Proc Natl Acad Sci USA. 1997;94:14048–14053. doi: 10.1073/pnas.94.25.14048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Parent M B, McGaugh J L. Brain Res. 1994;661:97–103. doi: 10.1016/0006-8993(94)91186-x. [DOI] [PubMed] [Google Scholar]
- 30.Liang K C, Hon W, Davis M. Behav Neurosci. 1994;108:241–253. doi: 10.1037//0735-7044.108.2.241. [DOI] [PubMed] [Google Scholar]
- 31.Roozendaal B, McGaugh J L. Europ J Neurosci. 1997;9:76–83. doi: 10.1111/j.1460-9568.1997.tb01355.x. [DOI] [PubMed] [Google Scholar]
- 32.Ikegaya Y, Saito H, Abe K. Brain Res. 1995;671:351–354. doi: 10.1016/0006-8993(94)01403-5. [DOI] [PubMed] [Google Scholar]
- 33.Packard M G, Cahill L, McGaugh J L. Proc Natl Acad Sci USA. 1994;91:8477–8481. doi: 10.1073/pnas.91.18.8477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Squire L R, Alvarez P. Curr Opin Neurobiol. 1995;5:169–177. doi: 10.1016/0959-4388(95)80023-9. [DOI] [PubMed] [Google Scholar]