Abstract
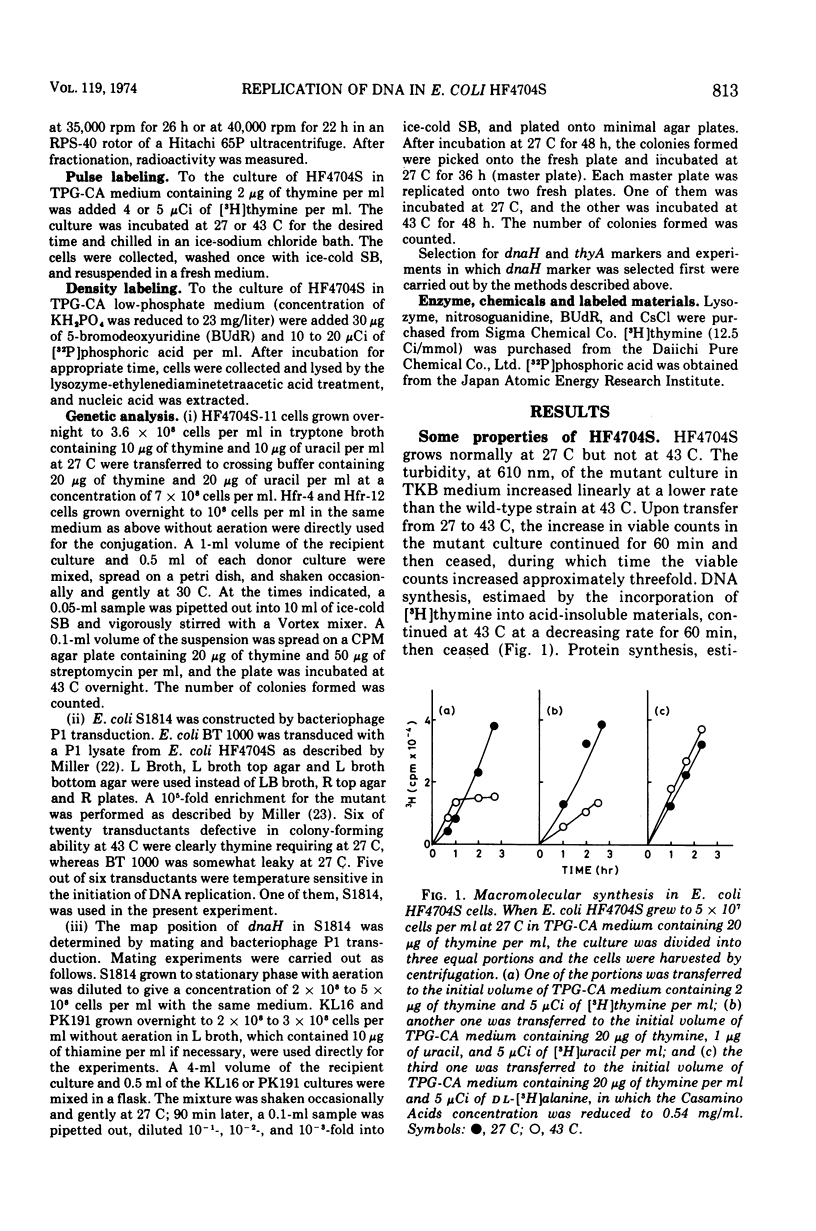
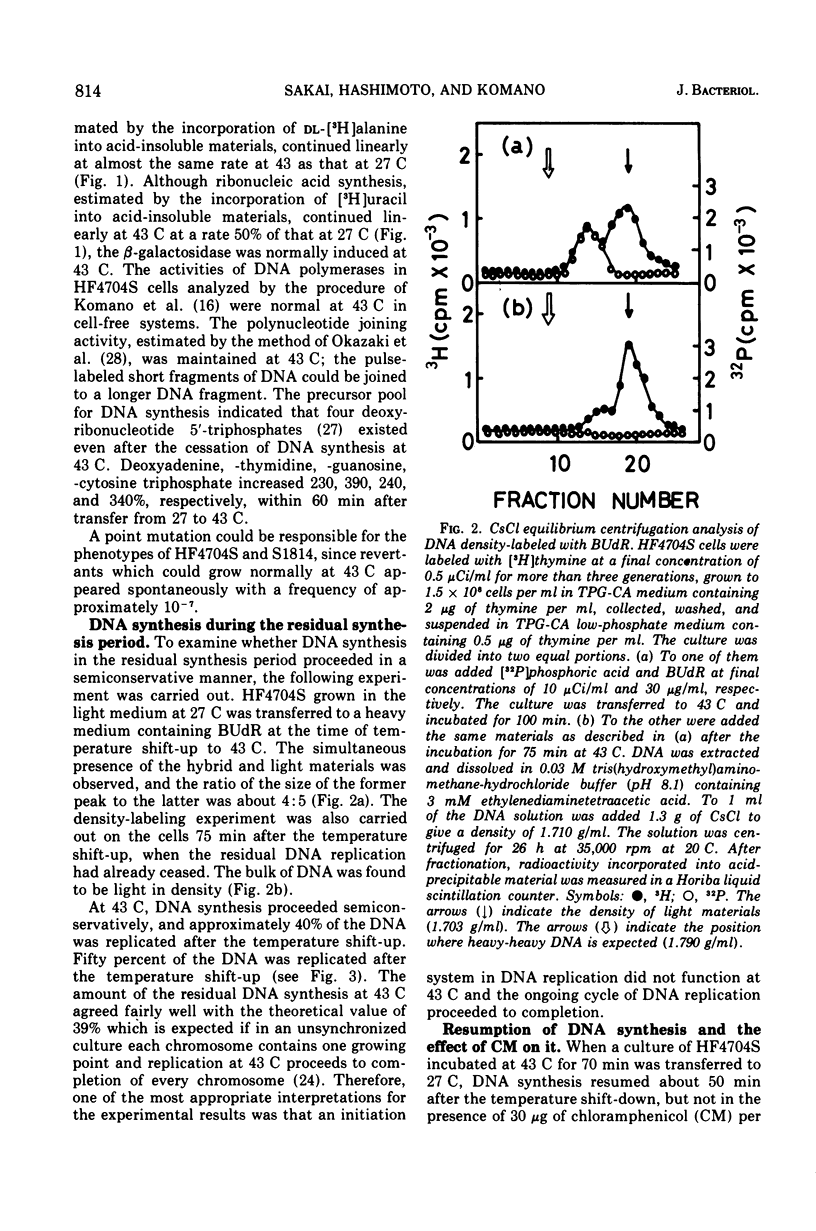
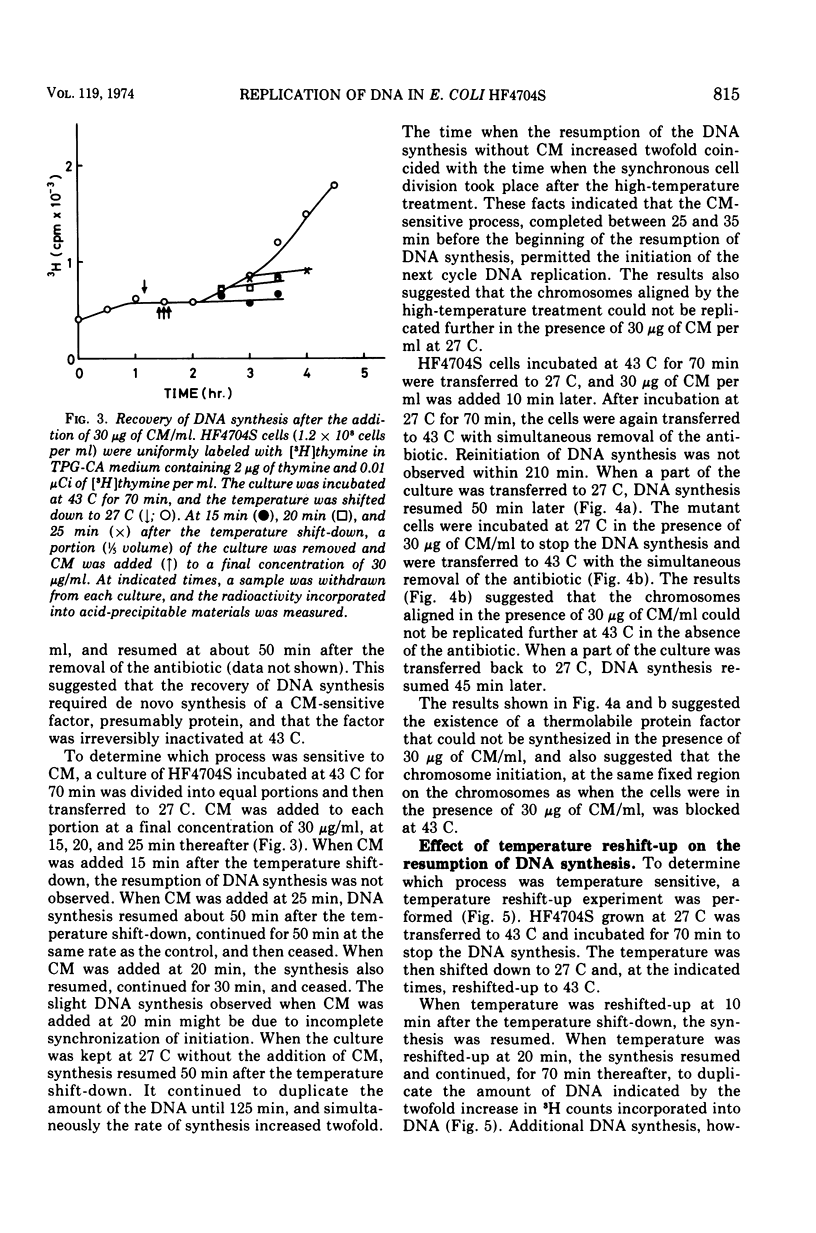
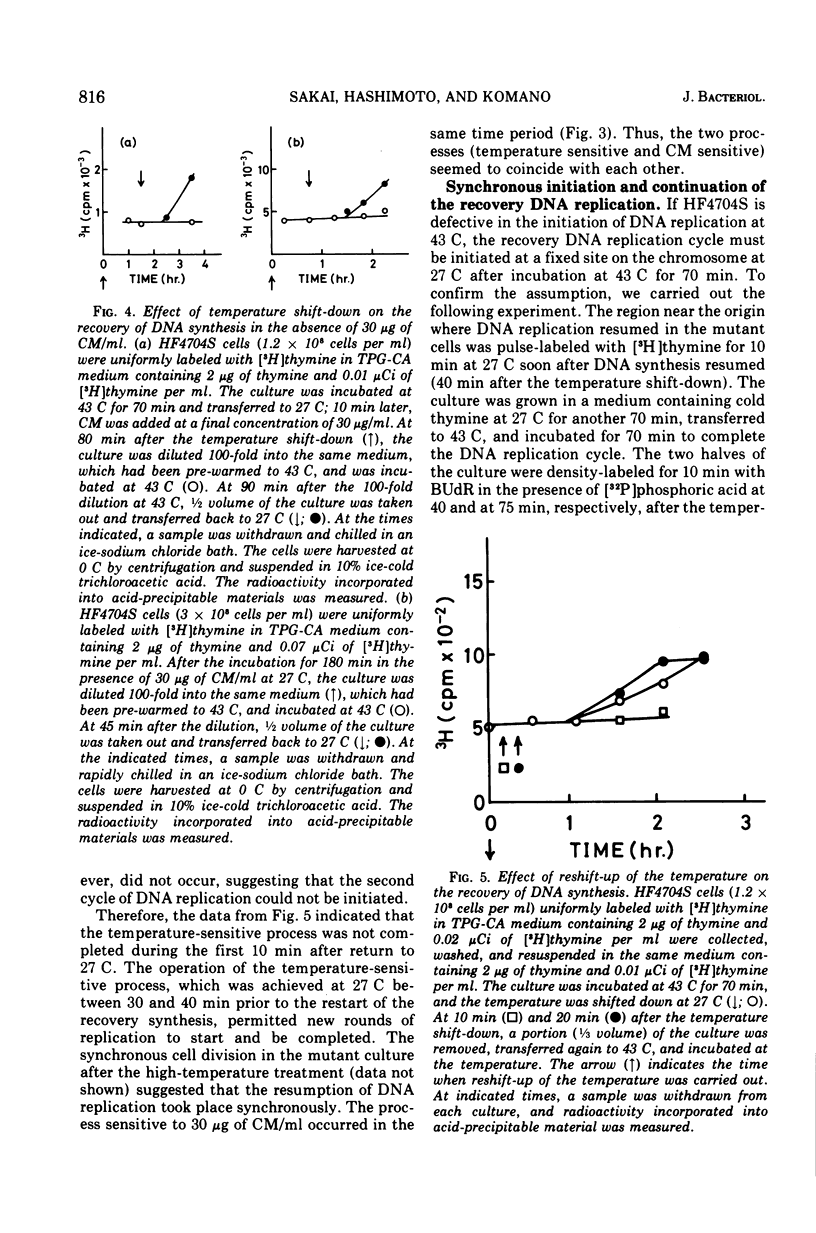
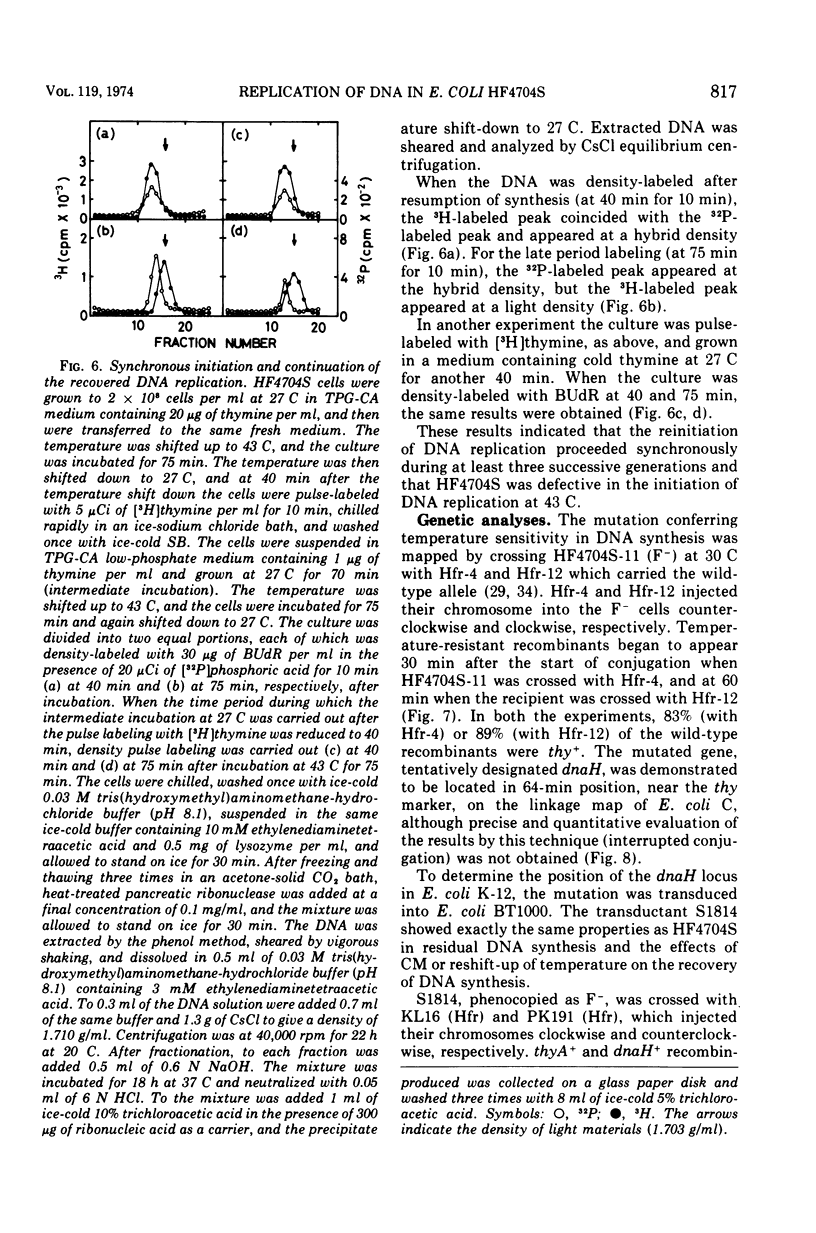
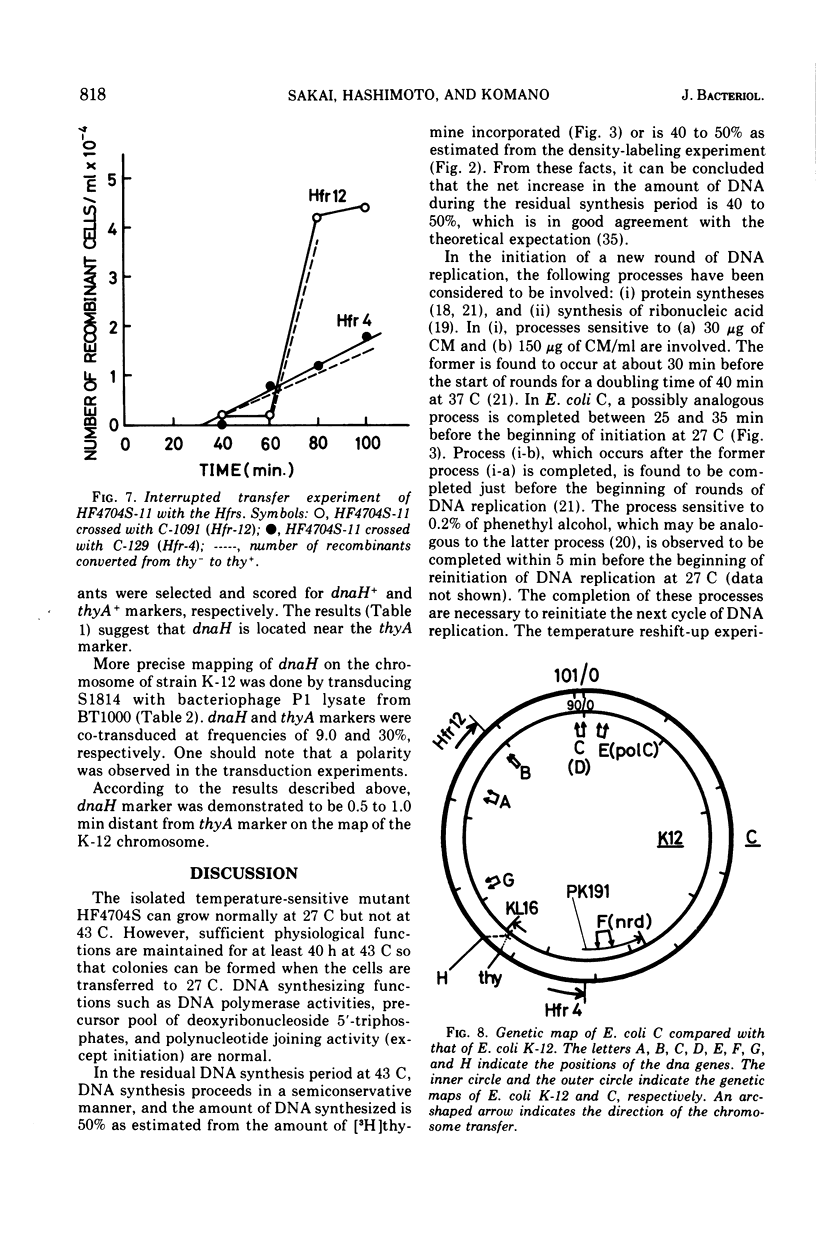
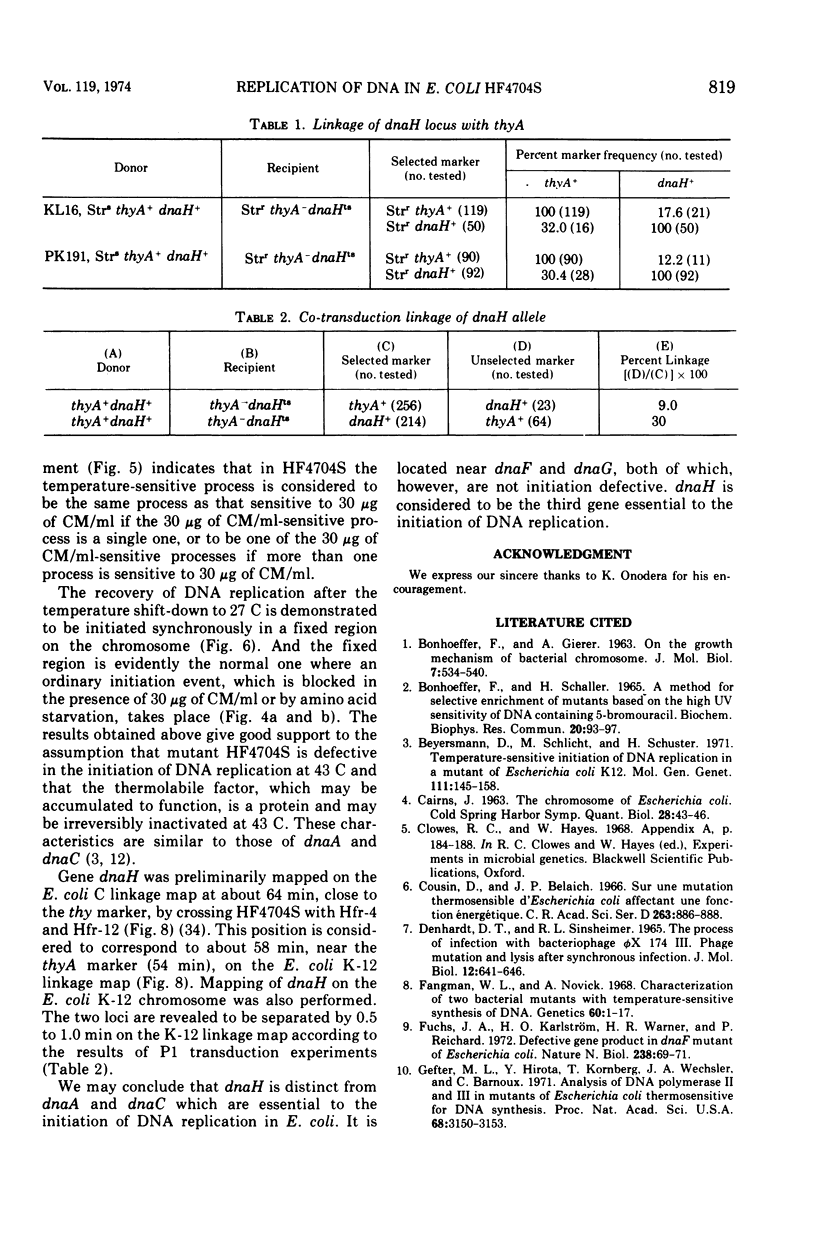
An Escherichia coli HF4704S mutant temperature sensitive in deoxyribonucleic acid (DNA) synthesis and different from any previously characterized mutant was isolated. The mutated gene in this strain was designated dnaH. The mutant could grow normally at 27 C but not at 43 C, and DNA synthesis continued for an hour at a decreasing rate and then ceased. After temperature shift-up, the increased amount of DNA was 40 to 50%. When the culture was incubated at 43 C for 70 min and then transferred to 27 C, DNA synthesis resumed after about 50 min, initiating synchronously at a fixed region on the bacterial chromosome. The initiation step in DNA replication sensitive to 30 μg of chloramphenicol per ml occurs synchronously before the resumption of DNA replication after the temperature shift-down, being completed about 30 min before the start of DNA replication. When the cells incubated at 27 C in the presence of 30 μg of chloramphenicol per ml after the temperature shift-down to 27 C were transferred to 43 C with simultaneous removal of the antibiotic, no resumption of DNA replication was observed. When the culture was returned to 43 C after being released from high-temperature inhibition at 30 min before the start of DNA replication, no recovery replication was observed; whereas at 20 min, the recovery of replication was observed. These results indicated that HF4704S was temperature sensitive in the initiation of DNA replication. Analysis of HF4704S, by an interrupted conjugation experiment, indicated that gene dnaH was located at about 64 min on the E. coli C linkage map. In E. coli S1814 (a K-12 derivative), which was a dnaHts transductant from HF4704S (C strain) with phage P1, the mutated gene (dnaH) was demonstrated to be closely linked to the thyA marker by conjugation and P1 transduction experiments and to be distinct from genes dnaA through dnaG.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BONHOEFFER F., GIERER A. ON THE GROWTH MECHANISM OF THE BACTERIAL CHROMOSOME. J Mol Biol. 1963 Nov;7:534–540. doi: 10.1016/s0022-2836(63)80100-x. [DOI] [PubMed] [Google Scholar]
- BONHOEFFER F., SCHALLER H. A METHOD FOR SELECTIVE ENRICHMENT OF MUTANTS BASED ON THE HIGH UV SENSITIVITY OF DNA CONTAINING 5-BROMOURACIL. Biochem Biophys Res Commun. 1965 Jun 18;20:93–97. [PubMed] [Google Scholar]
- Beyersmann D., Schlicht M., Schuster H. Temperature-sensitive initiation of DNA replication in a mutant of Escherichia coli K12. Mol Gen Genet. 1971;111(2):145–158. doi: 10.1007/BF00267789. [DOI] [PubMed] [Google Scholar]
- Cousin D., Belaïch J. P. Sur une mutatio thermosensible d'Escherichia coli affectant une fonction énergétique. C R Acad Sci Hebd Seances Acad Sci D. 1966 Sep 19;263(12):886–888. [PubMed] [Google Scholar]
- Denhardt D. T., Sinsheimer R. L. The process of infection with bacteriophage phi-X174. 3. Phage maturation and lysis after synchronized infection. J Mol Biol. 1965 Jul;12(3):641–646. doi: 10.1016/s0022-2836(65)80318-7. [DOI] [PubMed] [Google Scholar]
- Fangman W. L., Novick A. Characterization of two bacterial mutants with temperature-sensitive synthesis of DNA. Genetics. 1968 Sep;60(1):1–17. doi: 10.1093/genetics/60.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fuchs J. A., Karlström H. O., Warner H. R., Reichard P. Defective gene product in dnaF mutant of Escherichia coli. Nat New Biol. 1972 Jul 19;238(81):69–71. doi: 10.1038/newbio238069a0. [DOI] [PubMed] [Google Scholar]
- Gefter M. L., Hirota Y., Kornberg T., Wechsler J. A., Barnoux C. Analysis of DNA polymerases II and 3 in mutants of Escherichia coli thermosensitive for DNA synthesis. Proc Natl Acad Sci U S A. 1971 Dec;68(12):3150–3153. doi: 10.1073/pnas.68.12.3150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hewitt R., Billen D. Reorientation of chromosome replication after exposure to ultraviolet light of Escherichia coli. J Mol Biol. 1965 Aug;13(1):40–53. doi: 10.1016/s0022-2836(65)80078-x. [DOI] [PubMed] [Google Scholar]
- Hirota Y., Mordoh J., Jacob F. On the process of cellular division in Escherichia coli. 3. Thermosensitive mutants of Escherichia coli altered in the process of DNA initiation. J Mol Biol. 1970 Nov 14;53(3):369–387. doi: 10.1016/0022-2836(70)90072-0. [DOI] [PubMed] [Google Scholar]
- Hirota Y., Ryter A., Jacob F. Thermosensitive mutants of E. coli affected in the processes of DNA synthesis and cellular division. Cold Spring Harb Symp Quant Biol. 1968;33:677–693. doi: 10.1101/sqb.1968.033.01.077. [DOI] [PubMed] [Google Scholar]
- KOHIYAMA M., LANFROM H., BRENNER S., JACOB F. MODIFICATIONS DE FONCTIONS INDISPENSABLES CHEZ DES MUTANTS THERMOSENSIBLES D'ESCHERICHIA COLI. SUR UNE MUTATION EMP ECHANT LA R'EPLICATION DU CHROMOSOME BACT'ERIEN. C R Hebd Seances Acad Sci. 1963 Sep 23;257:1979–1981. [PubMed] [Google Scholar]
- Kohiyama M., Cousin D., Ryter A., Jacob F. Mutants thermosensibles d'Escherichia coli K 12. I. Isolement et caractérisation rapide. Ann Inst Pasteur (Paris) 1966 Apr;110(4):465–486. [PubMed] [Google Scholar]
- Kuempel P. L. Temperature-sensitive initiation of chromosome replication in a mutant of Escherichia coli. J Bacteriol. 1969 Dec;100(3):1302–1310. doi: 10.1128/jb.100.3.1302-1310.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LARK C., LARK K. G. EVIDENCE FOR TWO DISTINCT ASPECTS OF THE MECHANISM REGULATING CHROMOSOME REPLICATION IN ESCHERICHIA COLI. J Mol Biol. 1964 Oct;10:120–136. doi: 10.1016/s0022-2836(64)80032-2. [DOI] [PubMed] [Google Scholar]
- Lark K. G. Evidence for the direct involvement of RNA in the initiation of DNA replication in Escherichia coli 15T. J Mol Biol. 1972 Feb 28;64(1):47–60. doi: 10.1016/0022-2836(72)90320-8. [DOI] [PubMed] [Google Scholar]
- Lark K. G., Lark C. Regulation of chromosome replication in Escherichia coli: a comparison of the effects of phenethyl alcohol treatment with those of amino acid starvation. J Mol Biol. 1966 Sep;20(1):9–19. doi: 10.1016/0022-2836(66)90113-6. [DOI] [PubMed] [Google Scholar]
- Lark K. G., Renger H. Initiation of DNA replication in Escherichia coli 15T-: chronological dissection of three physiological processes required for initiation. J Mol Biol. 1969 Jun 14;42(2):221–235. doi: 10.1016/0022-2836(69)90039-4. [DOI] [PubMed] [Google Scholar]
- MAALOE O., HANAWALT P. C. Thymine deficiency and the normal DNA replication cycle. I. J Mol Biol. 1961 Apr;3:144–155. doi: 10.1016/s0022-2836(61)80041-7. [DOI] [PubMed] [Google Scholar]
- NAGATA T. The molecular synchrony and sequential replication of DNA in Escherichia coli. Proc Natl Acad Sci U S A. 1963 Apr;49:551–559. doi: 10.1073/pnas.49.4.551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neuhard J., Munch-Petersen A. Studies on the acid-soluble nucleotide pool in thymine-requiring mutants of Escherichia coli during thymine starvation. II. Changes in the amounts of deoxycytidine triphosphate and deoxyadenosine triphosphate in Escherichia coli 15 T-A-U. Biochim Biophys Acta. 1966 Jan 18;114(1):61–71. doi: 10.1016/0005-2787(66)90253-x. [DOI] [PubMed] [Google Scholar]
- Okazaki R., Okazaki T., Sakabe K., Sugimoto K., Sugino A. Mechanism of DNA chain growth. I. Possible discontinuity and unusual secondary structure of newly synthesized chains. Proc Natl Acad Sci U S A. 1968 Feb;59(2):598–605. doi: 10.1073/pnas.59.2.598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sasaki I., Bertani G. Growth abnormalities in Hfr derivatives of Escherichia coli strain C. J Gen Microbiol. 1965 Sep;40(3):365–376. doi: 10.1099/00221287-40-3-365. [DOI] [PubMed] [Google Scholar]
- Schekman R., Wickner W., Westergaard O., Brutlag D., Geider K., Bertsch L. L., Kornberg A. Initiation of DNA synthesis: synthesis of phiX174 replicative form requires RNA synthesis resistant to rifampicin. Proc Natl Acad Sci U S A. 1972 Sep;69(9):2691–2695. doi: 10.1073/pnas.69.9.2691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wechsler J. A., Gross J. D. Escherichia coli mutants temperature-sensitive for DNA synthesis. Mol Gen Genet. 1971;113(3):273–284. doi: 10.1007/BF00339547. [DOI] [PubMed] [Google Scholar]
- Wickner R. B., Wright M., Wickner S., Hurwitz J. Conversion of phiX174 and fd single-stranded DNA to replicative forms in extracts of Escherichia coli. Proc Natl Acad Sci U S A. 1972 Nov;69(11):3233–3237. doi: 10.1073/pnas.69.11.3233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wiman M., Bertani G., Kelly B., Sasaki I. Genetic map of Escherichia coli strain C. Mol Gen Genet. 1970;107(1):1–31. doi: 10.1007/BF00433220. [DOI] [PubMed] [Google Scholar]
- Yoshikawa H. DNA synthesis during germination of Bacillus subtilis spores. Proc Natl Acad Sci U S A. 1965 Jun;53(6):1476–1483. doi: 10.1073/pnas.53.6.1476. [DOI] [PMC free article] [PubMed] [Google Scholar]


