Abstract
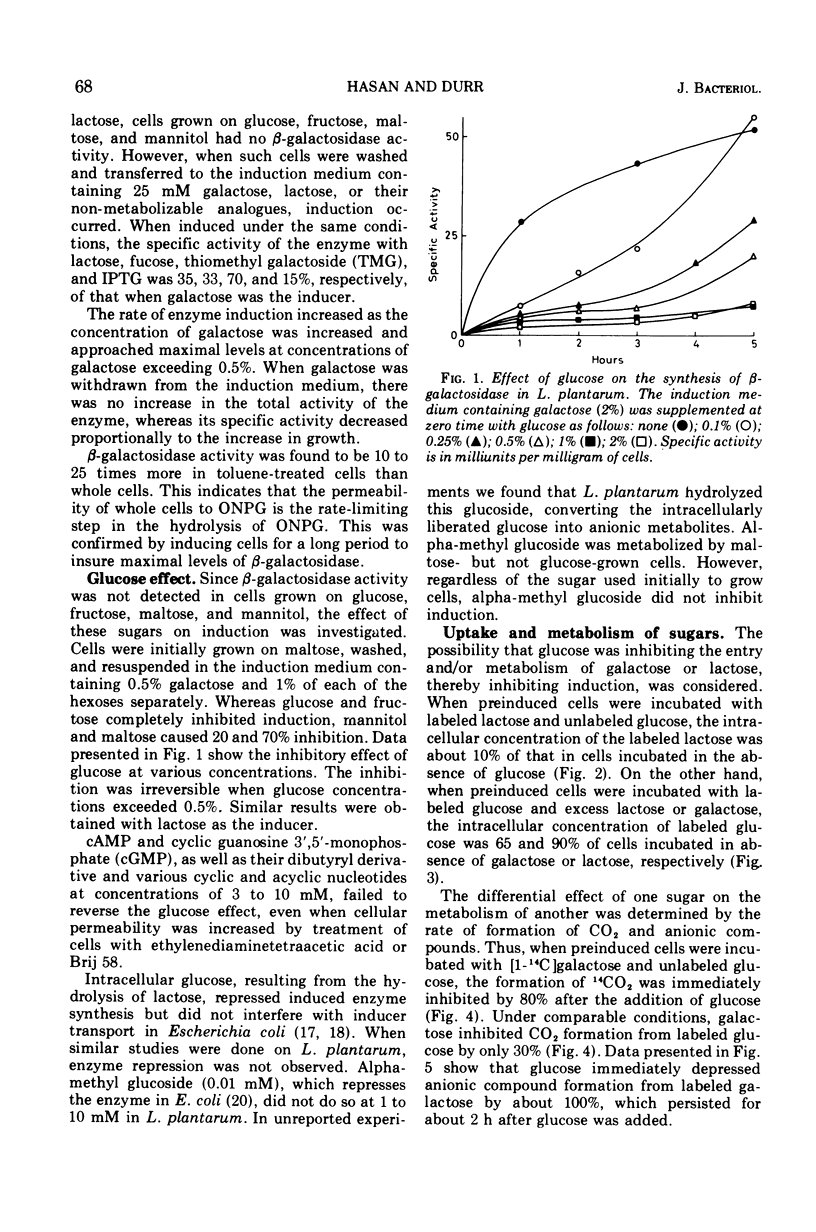
β-galactosidase (β-galactoside galactohydrolase, EC 3.2.1.23) is inducible in Lactobacillus plantarum by d-galactose or thiomethyl galactoside, and to a much lesser extent by lactose, isopropyl thiomethyl galactoside, and d-fucose. Isopropyl thiomethyl galactoside is a competitive inhibitor of the enzyme with a Ki of 4.2 mM. The Km of the crude enzyme for o-nitrophenyl β-d-galactoside is 0.87 mM. Induction also requires a source of energy and amino acids. Chloramphenicol and actinomycin D inhibited induction. d-Glucose, d-fructose and to a lesser extent maltose and d-mannitol inhibited enzyme synthesis. Methyl-alpha-d-glucopyranoside was not inhibitory. Glucose exerts its effect through its ability to exclude galactose or lactose entry into the cell. The uptake of lactose and the metabolism of galactose by preinduced cells is severely inhibited by glucose. But neither galactose nor lactose severely affected the uptake of glucose by preinduced cells. Thus, glucose acts through catabolite inhibition, i.e., transport of inducer rather than repression through transcription or related mechanisms. This is supported by the inability of cyclic nucleotides to relieve the inhibition produced by glucose or to stimulate induction. Furthermore, intracellularly produced glucose did not inhibit enzyme synthesis. Acetate and mevalonate, the precursors of membrane lipids, stimulate induction independently of their effect on growth. Homobiotin partially abolished the acetate effect but did not inhibit induction or growth.
Full text
PDF






Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Adhya S., Echols H. Glucose effect and the galactose enzymes of Escherichia coli: correlation between glucose inhibition of induction and inducer transport. J Bacteriol. 1966 Sep;92(3):601–608. doi: 10.1128/jb.92.3.601-608.1966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CHAKRAVORTY M. INDUCTION AND REPRESSION OF L-ARABINOSE ISOMERASE IN LACTOBACILLUS PLANTARUM. Biochim Biophys Acta. 1964 Apr 6;85:152–161. doi: 10.1016/0926-6569(64)90175-0. [DOI] [PubMed] [Google Scholar]
- CRESSON E. L., FOLKERS K., HOFFMAN C. H., MACRAE G. D., SKEGGS H. R., WOLF D. E., WRIGHT L. D. Discovery of a new acetate-replacing factor. J Bacteriol. 1956 Oct;72(4):519–524. doi: 10.1128/jb.72.4.519-524.1956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chakravorty M. Metabolism of mannitol and induction of mannitol 1-phosphate dehydrogenase in Lactobacillus plantarum. J Bacteriol. 1964 May;87(5):1246–1248. doi: 10.1128/jb.87.5.1246-1248.1964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DURR I. F., SHWAYRI A. N. METABOLISM OF MEVALONIC ACID BY LACTOBACILLUS PLANTARUM. J Bacteriol. 1964 Aug;88:361–366. doi: 10.1128/jb.88.2.361-366.1964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Crombrugghe B., Perlman R. L., Varmus H. E., Pastan I. Regulation of inducible enzyme synthesis in Escherichia coli by cyclic adenosine 3', 5'-monophosphate. J Biol Chem. 1969 Nov 10;244(21):5828–5835. [PubMed] [Google Scholar]
- Durr I. F. The biosynthesis of squalene and sterols by the adipose tissue of rat, sheep and man. Biochem J. 1966 Jan;98(1):317–320. doi: 10.1042/bj0980317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fox C. F. A lipid requirement for induction of lactose transport in Escherichia coli. Proc Natl Acad Sci U S A. 1969 Jul;63(3):850–855. doi: 10.1073/pnas.63.3.850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holden J. T., Hild O., Wong-Leung Y. L., Rouser G. Reduced lipid content as the basis for defective amino acid accumulation capacity in pantothenate- and biotin-deficient Lactobacillus plantarum. Biochem Biophys Res Commun. 1970 Jul 13;40(1):123–128. doi: 10.1016/0006-291x(70)91055-7. [DOI] [PubMed] [Google Scholar]
- KOPPEL J. L., PORTER C. J., CROCKER B. F. The mechanism of the synthesis of enzymes. I. Development of a system suitable for studying this phenomenon. J Gen Physiol. 1953 May;36(5):703–722. doi: 10.1085/jgp.36.5.703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LEDERBERG J. The beta-d-galactosidase of Escherichia coli, strain K-12. J Bacteriol. 1950 Oct;60(4):381–392. doi: 10.1128/jb.60.4.381-392.1950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- London J., Meyer E. Y. Malate utilization by a group D Streptococcus: physiological properties and purification of an inducible malic enzyme. J Bacteriol. 1969 May;98(2):705–711. doi: 10.1128/jb.98.2.705-711.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MAGASANIK B. Catabolite repression. Cold Spring Harb Symp Quant Biol. 1961;26:249–256. doi: 10.1101/sqb.1961.026.01.031. [DOI] [PubMed] [Google Scholar]
- MAKMAN R. S., SUTHERLAND E. W. ADENOSINE 3',5'-PHOSPHATE IN ESCHERICHIA COLI. J Biol Chem. 1965 Mar;240:1309–1314. [PubMed] [Google Scholar]
- McGinnis J. F., Paigen K. Catabolite inhibition: a general phenomenon in the control of carbohydrate utilization. J Bacteriol. 1969 Nov;100(2):902–913. doi: 10.1128/jb.100.2.902-913.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGinnis J. F., Paigen K. Site of catabolite inhibition of carbohydrate metabolism. J Bacteriol. 1973 May;114(2):885–887. doi: 10.1128/jb.114.2.885-887.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pastan I., Perlman R. L. Repression of beta-galactosidase synthesis by glucose in phosphotransferase mutants of Escherichia coli. Repression in the absence of glucose phosphorylation. J Biol Chem. 1969 Nov 10;244(21):5836–5842. [PubMed] [Google Scholar]
- Sahyoun N., Durr I. F. Evidence against the presence of 3',5'-cyclic adenosine monophosphate and relevant enzymes in Lactobacillus plantarum. J Bacteriol. 1972 Oct;112(1):421–426. doi: 10.1128/jb.112.1.421-426.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waller J. R., Lichstein H. C. Biotin transport and accumulation by cells of Lactobacillus plantarum. I. General properties of the system. J Bacteriol. 1965 Oct;90(4):843–852. doi: 10.1128/jb.90.4.843-852.1965. [DOI] [PMC free article] [PubMed] [Google Scholar]


