Abstract
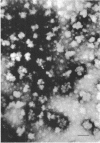
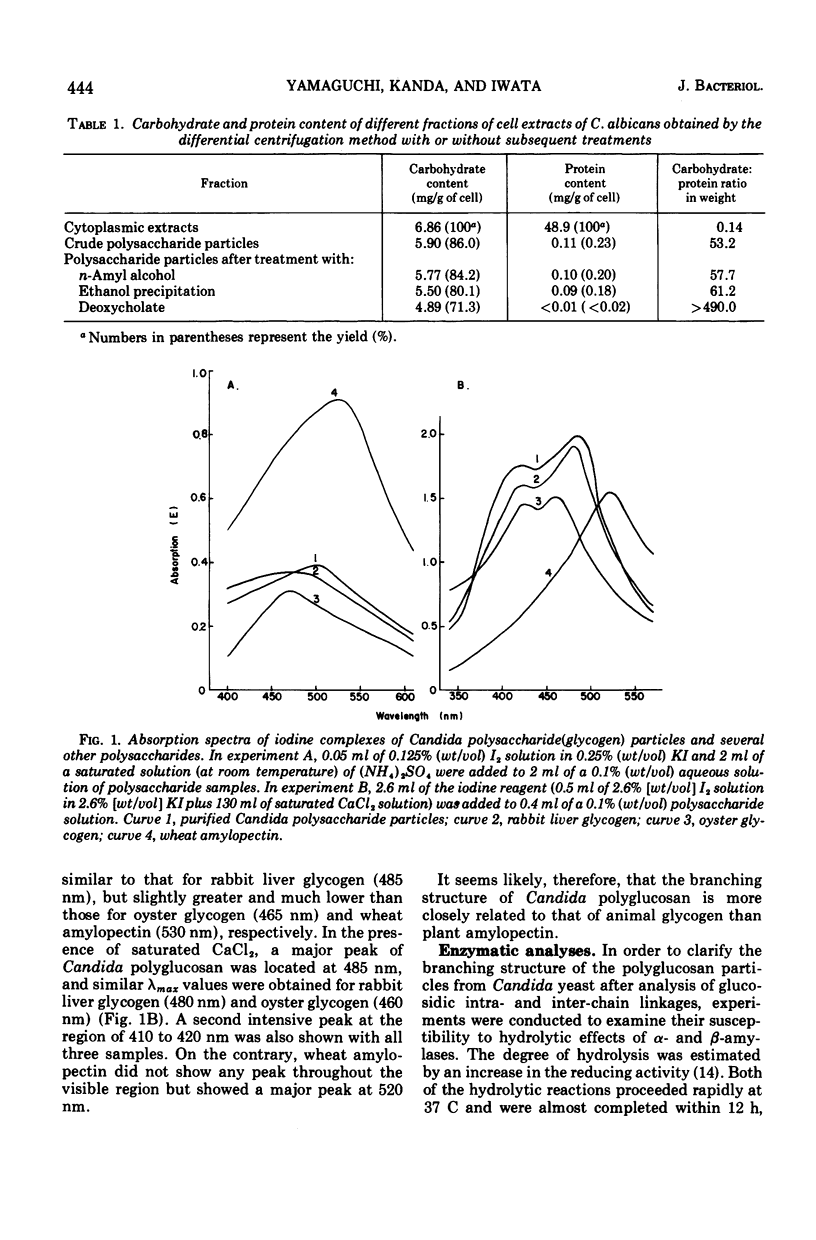
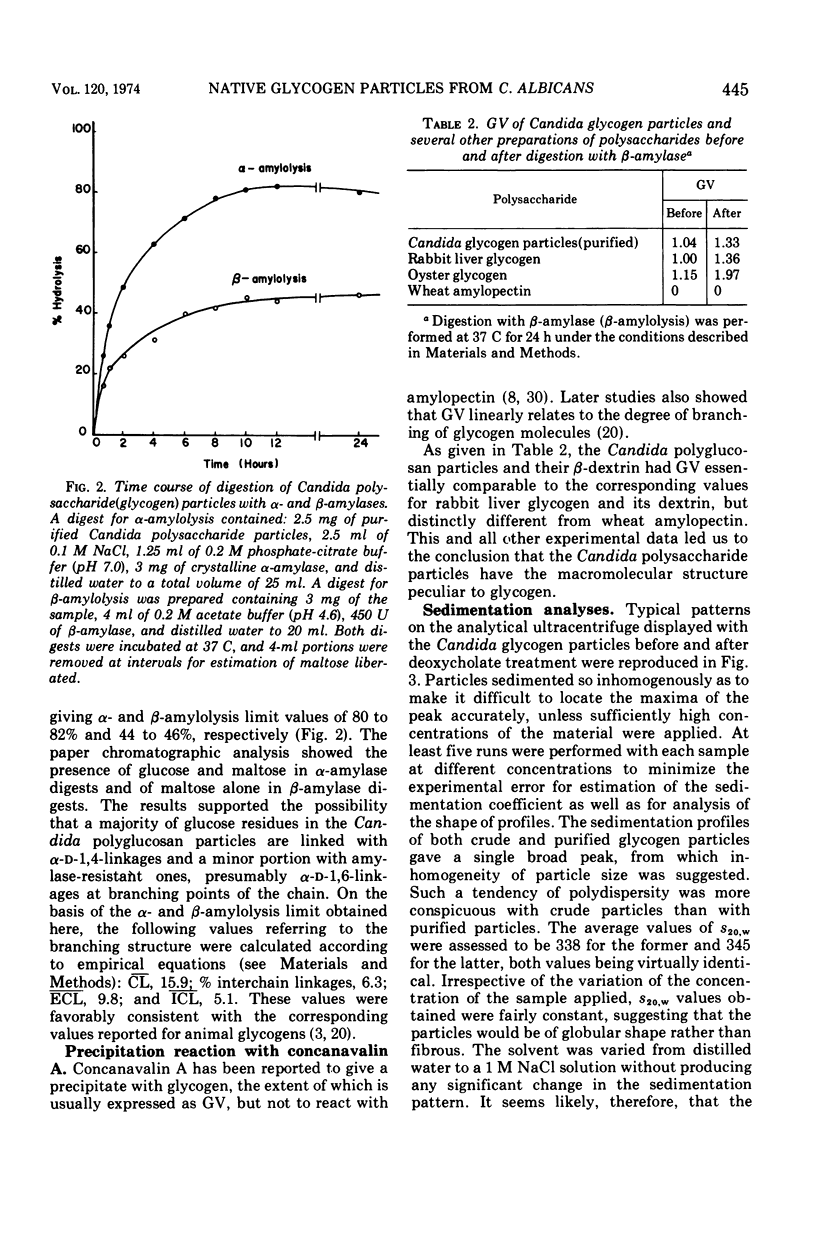
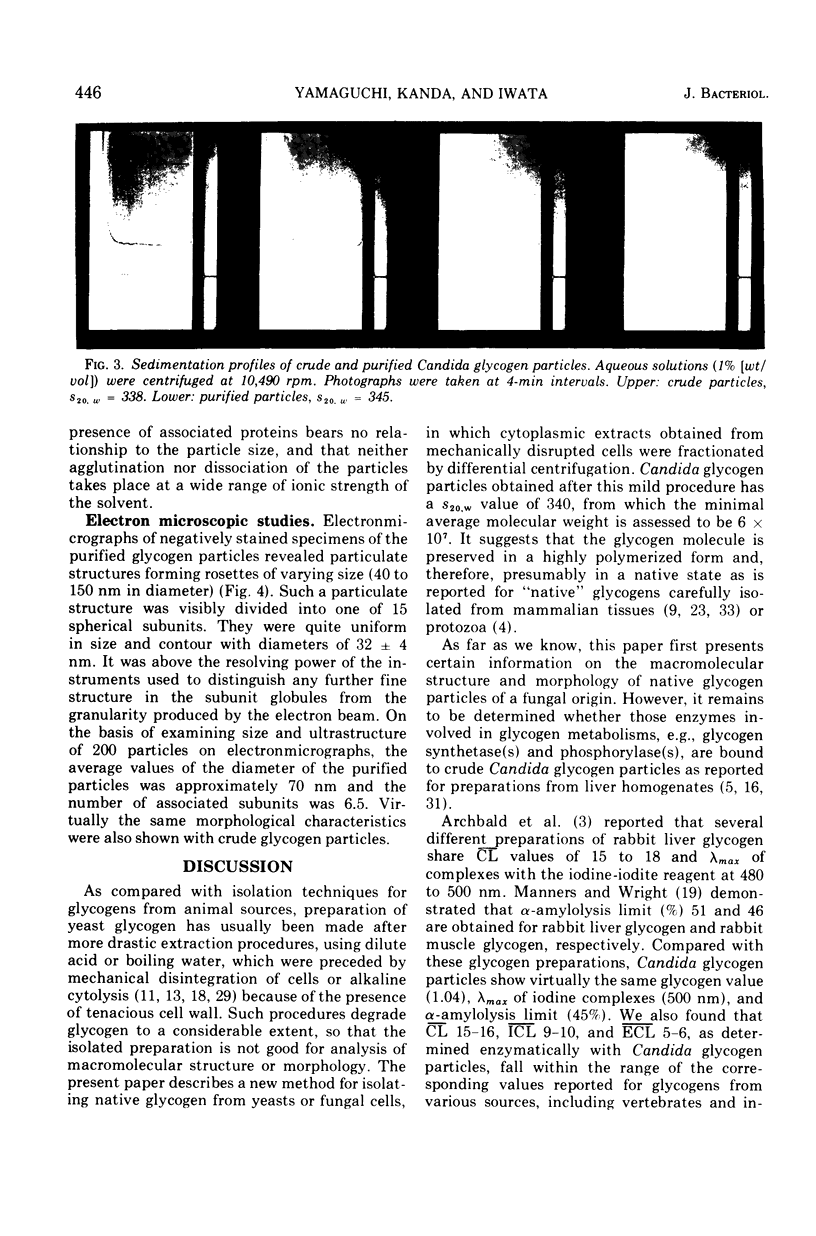
A polysaccharide-rich particulate fraction was isolated from cytoplasmic extracts of Candida albicans by a procedure using differential centrifugation. The polysaccharide particles obtained after purification with deoxycholate treatment were essentially free of nitrogen and were identified chemically as polyglucosan, in which the glucosidic links were of alpha type. Both the response to amylolytic enzymes and the spectral characteristics of the iodine complexes of the polysaccharide particles were similar to those of rabbit liver glycogen. They also precipitated with concanavalin A, the glycogen value being assessed at 1.04. These data strongly indicated that the polysaccharide particles have the macromolecular structure characteristic of glycogen. The sedimentation analysis revealed that they were polydisperse, with a weight average sedimentation coefficient of 340S. In negatively stained specimens, the glycogen particles were seen to form rosette-like structures consisting of a complex unit 40 to 150 nm in diameter. Such complex particles were composed of smaller globules that were fairly uniform in size with an average diameter of 32 nm.
Full text
PDF





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Barber A. A., Harris W. W., Padilla G. M. Studies of native glycogen isolated from synchronized Tetrahymena pyriformis (HSM). J Cell Biol. 1965 Nov;27(2):281–292. doi: 10.1083/jcb.27.2.281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barber A. A., Orrell S. A., Jr, Bueding E. Association of enzymes with rat liver glycogen isolated by rate-zonal centrifugation. J Biol Chem. 1967 Sep 25;242(18):4040–4044. [PubMed] [Google Scholar]
- CHUNG C. W., NICKERSON W. J. Polysaccharide syntheses in growing yeasts. J Biol Chem. 1954 May;208(1):395–407. [PubMed] [Google Scholar]
- DROCHMANS P. [Morphology of glycogen. Electron microscopic study of the negative stains of particulate glycogen]. J Ultrastruct Res. 1962 Apr;6:141–163. doi: 10.1016/s0022-5320(62)90050-3. [DOI] [PubMed] [Google Scholar]
- HORNE R. W., GREVILLE G. D. Observations on ox-liver L-glutamate dehydrogenase with the electron microscope. J Mol Biol. 1963 Jun;6:506–509. doi: 10.1016/s0022-2836(63)80062-5. [DOI] [PubMed] [Google Scholar]
- KATZEN H. M., STETTEN D., Jr, STETTEN M. R. Metabolic inhomogeneity of glycogen as a function of molecular weight. J Biol Chem. 1956 Oct;222(2):587–599. [PubMed] [Google Scholar]
- KRISMAN C. R. A method for the colorimetric estimation of glycogen with iodine. Anal Biochem. 1962 Jul;4:17–23. doi: 10.1016/0003-2697(62)90014-3. [DOI] [PubMed] [Google Scholar]
- LELOIR L. F., GOLDEMBERG S. H. Synthesis of glycogen from uridine diphosphate glucose in liver. J Biol Chem. 1960 Apr;235:919–923. [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- MUNDKUR B. Electron microscopical studies of frozen-dried yeast. I. Localization of polysaccharides. Exp Cell Res. 1960 Jun;20:28–42. doi: 10.1016/0014-4827(60)90219-6. [DOI] [PubMed] [Google Scholar]
- McAnally R. A., Smedley-Maclean I. The synthesis of reserve carbohydrate by yeast: The nature of the insoluble carbohydrate. Biochem J. 1937 Jan;31(1):72–80. doi: 10.1042/bj0310072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SCHLAMOWITZ M. On the nature of rabbit liver glycogen. II. Iodine absorption spectrum. J Biol Chem. 1951 Jun;190(2):519–527. [PubMed] [Google Scholar]
- SMOGYI M. Notes on sugar determination. J Biol Chem. 1952 Mar;195(1):19–23. [PubMed] [Google Scholar]
- TREVELYAN W. E., PROCTER D. P., HARRISON J. S. Detection of sugars on paper chromatograms. Nature. 1950 Sep 9;166(4219):444–445. doi: 10.1038/166444b0. [DOI] [PubMed] [Google Scholar]
- Tata J. R. Subcellular redistribution of a liver alpha-glucan phosphorylase during alterations in glycogen content. Biochem J. 1964 Feb;90(2):284–292. doi: 10.1042/bj0900284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wanson J. C., Drochmans P. Rabbit skeletal muscle glycogen. A morphological and biochemical study of glycogen beta-particles isolated by the precipitation-centrifugation method. J Cell Biol. 1968 Jul;38(1):130–150. doi: 10.1083/jcb.38.1.130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamaguchi H., Iwata K. In vitro an in vivo protein synthesis in Candida albicans. I. Isolation and protein synthesis of polysomes. Sabouraudia. 1970 Nov;8(3):177–188. [PubMed] [Google Scholar]