Abstract
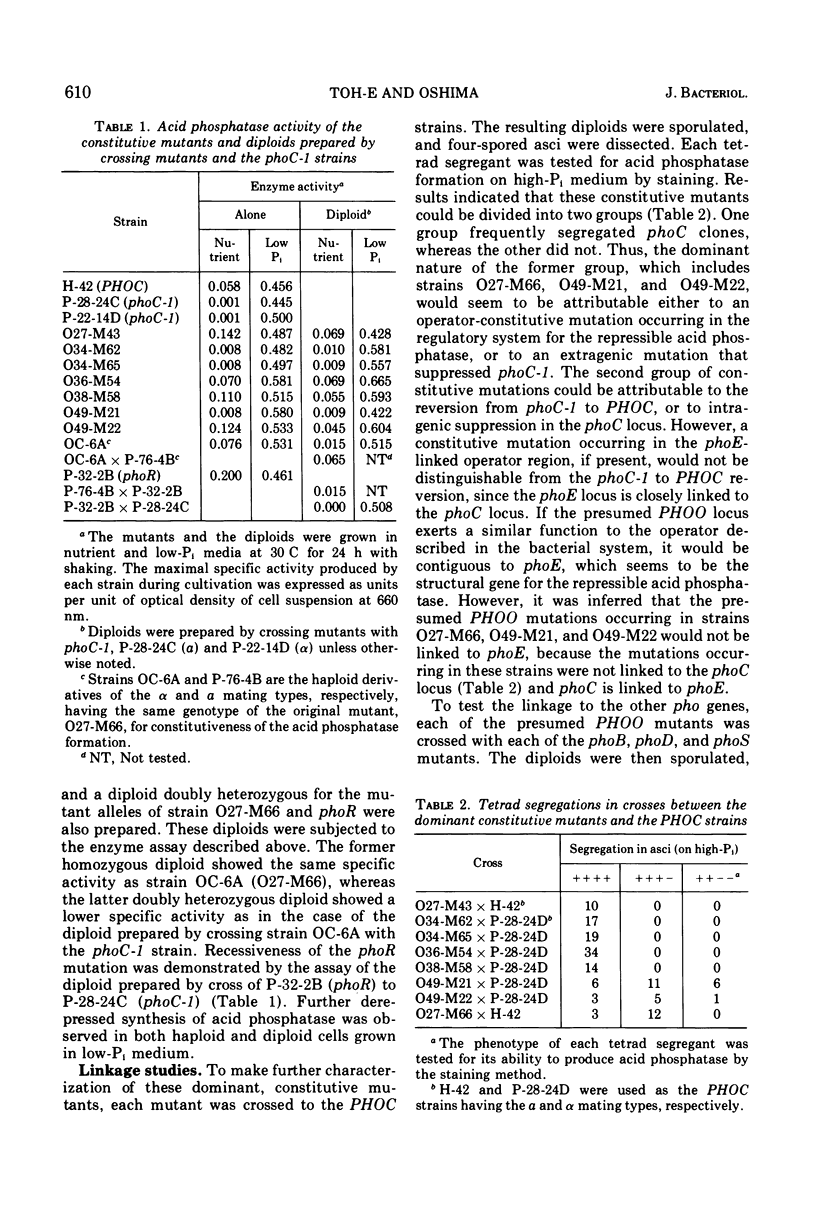
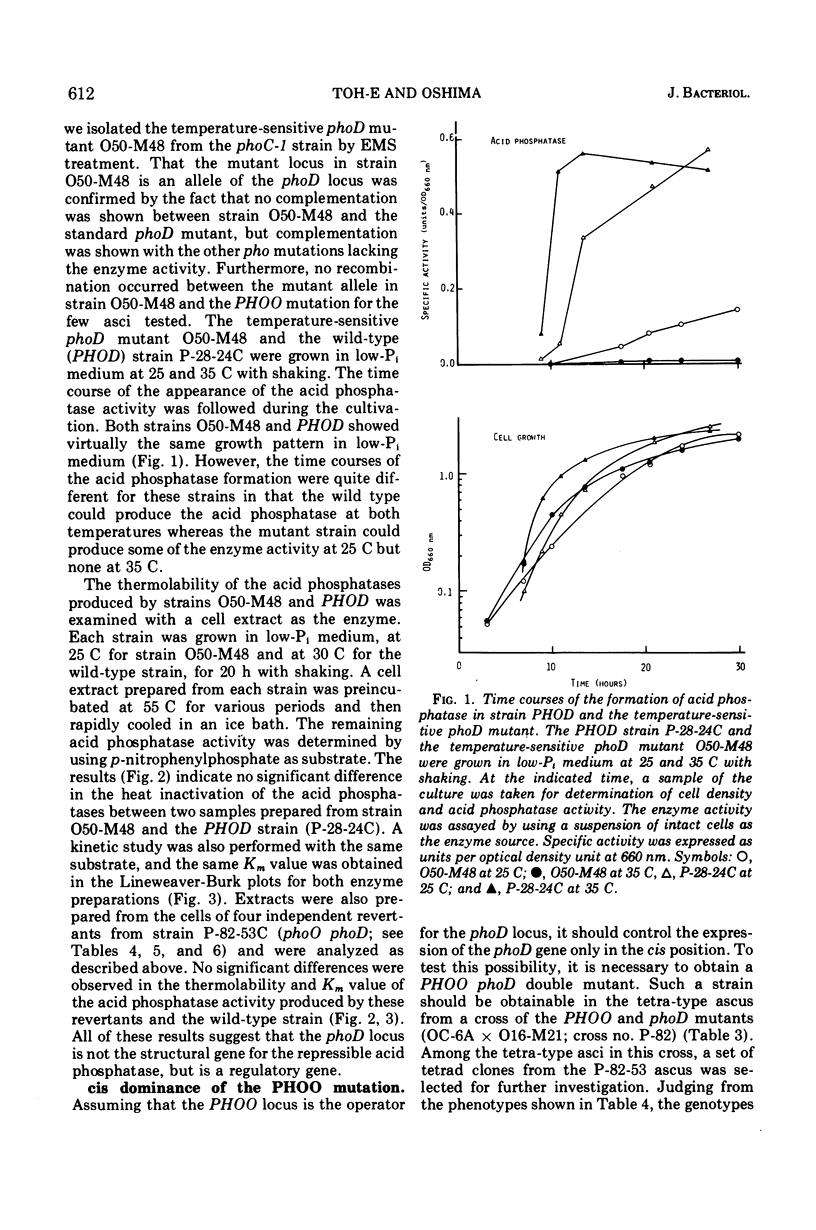
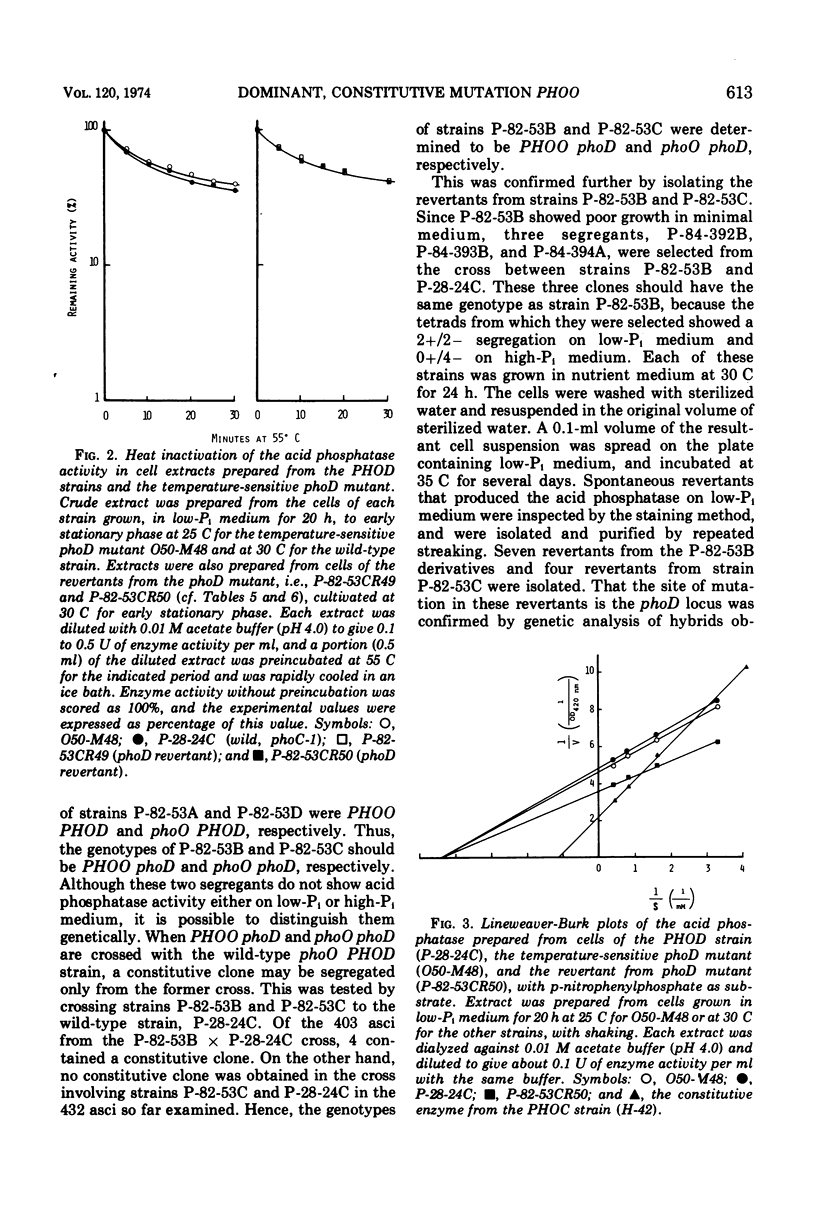
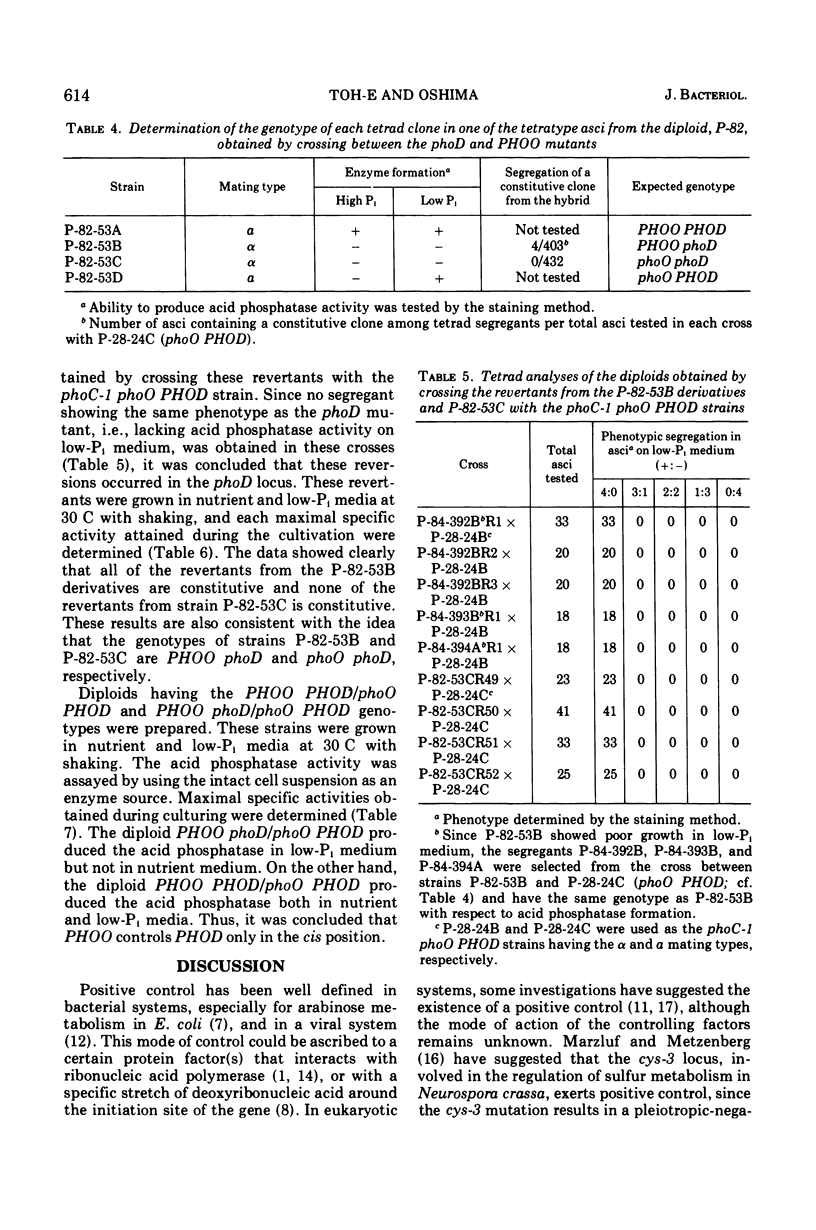
An apparent operator-constitutive mutation was discovered in the repressible acid phosphatase system in Saccharomyces cerevisiae. The site of mutation, designated PHOO, was found to be closely linked to the phoD locus. The mutant allele, PHOO, was semidominant over the wild-type allele and effective for the expression of the phoD gene in cis position. The phoD mutation gave rise to a defective phenotype for the formation of the repressible acid phosphatase. On the other hand, neither the repressible acid phosphatase activity in the cell-free extracts prepared from cells of the temperature-sensitive phoD mutant grown at 25 C, nor that of the revertants from the phoD mutants, could be distinguished from that of the wild-type strain with respect to thermolability and Km value for p-nitrophenylphosphate. These results strongly suggest that the phoD gene is not a structural gene, but a regulatory gene exerting positive control for the formation of repressible acid phosphatase. Close similarity between the apparent role of the phoO-PHOD gene cluster and that of the c-GAL4 gene cluster in the galactose system of S. cerevisiae could be inferred.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BLUM J. J. OBSERVATIONS ON THE ACID PHOSPHATASES OF EUGLENA GRACILIS. J Cell Biol. 1965 Feb;24:223–234. doi: 10.1083/jcb.24.2.223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bautz E. K., Bautz F. A., Dunn J. J. E. coli sigma factor: a positive control element in phage T4 development. Nature. 1969 Sep 6;223(5210):1022–1024. doi: 10.1038/2231022a0. [DOI] [PubMed] [Google Scholar]
- Douglas H. C., Hawthorne D. C. Regulation of genes controlling synthesis of the galactose pathway enzymes in yeast. Genetics. 1966 Sep;54(3):911–916. doi: 10.1093/genetics/54.3.911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dunsmuir P., Hynes M. J. Temperature sensitive mutants affecting the activity and regulation of the acetamidase of Aspergillus nidulans. Mol Gen Genet. 1973 Jul 16;123(4):333–346. doi: 10.1007/BF00433650. [DOI] [PubMed] [Google Scholar]
- ECHOLS H., GAREN A., GAREN S., TORRIANI A. Genetic control of repression of alkaline phosphatase in E. coli. J Mol Biol. 1961 Aug;3:425–438. doi: 10.1016/s0022-2836(61)80055-7. [DOI] [PubMed] [Google Scholar]
- Eron L., Block R. Mechanism of initiation and repression of in vitro transcription of the lac operon of Escherichia coli. Proc Natl Acad Sci U S A. 1971 Aug;68(8):1828–1832. doi: 10.1073/pnas.68.8.1828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GAREN A., ECHOLS H. Genetic control of induction of alkaline phosphatase synthesis in E. coli. Proc Natl Acad Sci U S A. 1962 Aug;48:1398–1402. doi: 10.1073/pnas.48.8.1398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GAREN A., ECHOLS H. Properties of two regulating genes for alkaline phosphatase. J Bacteriol. 1962 Feb;83:297–300. doi: 10.1128/jb.83.2.297-300.1962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HORIUCHI T., HORIUCHI S., MIZUNO D. A possible negative feedback phenomenon controlling formation of alkaline phosphomonoesterase in Escherichia coli. Nature. 1959 May 30;183(4674):1529–1530. doi: 10.1038/1831529b0. [DOI] [PubMed] [Google Scholar]
- Herskowitz I. Control of gene expression in bacteriophage lambda. Annu Rev Genet. 1973;7:289–324. doi: 10.1146/annurev.ge.07.120173.001445. [DOI] [PubMed] [Google Scholar]
- Horvitz H. R. Polypeptide bound to the host RNA polymerase is specified by T4 control gene 33. Nat New Biol. 1973 Aug 1;244(135):137–140. doi: 10.1038/newbio244137a0. [DOI] [PubMed] [Google Scholar]
- Lindegren G., Hwang Y. L., Oshima Y., Lindegren C. C. Genetical mutants induced by ethyl methanesulfonate in Saccharomyces. Can J Genet Cytol. 1965 Sep;7(3):491–499. doi: 10.1139/g65-064. [DOI] [PubMed] [Google Scholar]
- Marzluf G. A., Metzenberg R. L. Positive control by the cys-3 locus in regulation of sulfur metabolism in Neurospora. J Mol Biol. 1968 Apr 28;33(2):423–437. doi: 10.1016/0022-2836(68)90199-x. [DOI] [PubMed] [Google Scholar]
- Metzenberg R. L. Genetic regulatory systems in Neurospora. Annu Rev Genet. 1972;6:111–132. doi: 10.1146/annurev.ge.06.120172.000551. [DOI] [PubMed] [Google Scholar]
- Miki T., Minami Z., Ikeda Y. The genetics of alkaline phosphatase formation in Bacillus subtilis. Genetics. 1965 Nov;52(5):1093–1100. doi: 10.1093/genetics/52.5.1093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nyc J. F. A repressible acid phosphatase in Neurospora crassa. Biochem Biophys Res Commun. 1967 Apr 20;27(2):183–188. doi: 10.1016/s0006-291x(67)80059-7. [DOI] [PubMed] [Google Scholar]
- Nyc J. F., Kadner R. J., Crocken B. J. A repressible alkaline phosphatase in Neurospora crassa. J Biol Chem. 1966 Apr 10;241(7):1468–1472. [PubMed] [Google Scholar]
- Polacco J. C., Gross S. R. The product of the leu-3 cistron as a regulatory element for the production of the leucine biosynthetic enzymes of Neurospora. Genetics. 1973 Jul;74(3):443–459. doi: 10.1093/genetics/74.3.443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SCHMIDT G., BARTSCH G., LAUMONT M. C., HERMAN T., LISS M. Acid phosphatase of bakers' yeast: an enzyme of the external cell surface. Biochemistry. 1963 Jan-Feb;2:126–131. doi: 10.1021/bi00901a022. [DOI] [PubMed] [Google Scholar]
- SUOMALAINEN H., LINKO M., OURA E. Changes in the phosphatase activity of Baker's yeast during the growth phase and location of the phosphatases in the yeast cell. Biochim Biophys Acta. 1960 Jan 29;37:482–490. doi: 10.1016/0006-3002(60)90505-9. [DOI] [PubMed] [Google Scholar]
- Schurr A., Yagil E. Regulation and characterization of acid and alkaline phosphatase in yeast. J Gen Microbiol. 1971 Mar;65(3):291–303. doi: 10.1099/00221287-65-3-291. [DOI] [PubMed] [Google Scholar]
- Thuriaux P., Ramos F., Piérard A., Grenson M., Wiame J. M. Regulation of the carbamoylphosphate synthetase belonging to the arginine biosynthetic pathway of Saccharomyces cerevisiae. J Mol Biol. 1972 Jun 20;67(2):277–287. doi: 10.1016/0022-2836(72)90241-0. [DOI] [PubMed] [Google Scholar]
- To-E A., Ueda Y., Kakimoto S. I., Oshima Y. Isolation and characterization of acid phosphatase mutants in Saccharomyces cerevisiae. J Bacteriol. 1973 Feb;113(2):727–738. doi: 10.1128/jb.113.2.727-738.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Valone J. A., Jr, Case M. E., Giles N. H. Constitutive mutants in a regulatory gene exerting positive control of quinic acid catabolism in Neurospora crassa. Proc Natl Acad Sci U S A. 1971 Jul;68(7):1555–1559. doi: 10.1073/pnas.68.7.1555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Willsky G. R., Bennett R. L., Malamy M. H. Inorganic phosphate transport in Escherichia coli: involvement of two genes which play a role in alkaline phosphatase regulation. J Bacteriol. 1973 Feb;113(2):529–539. doi: 10.1128/jb.113.2.529-539.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zimmermann F. K., Schmiedt I., ten Berge A. M. Dominance and recessiveness at the protein level in mutant x wildtype crosses in Sacchaomyces cerevisiae. Mol Gen Genet. 1969 Aug 15;104(4):321–330. doi: 10.1007/BF00334231. [DOI] [PubMed] [Google Scholar]


