Abstract
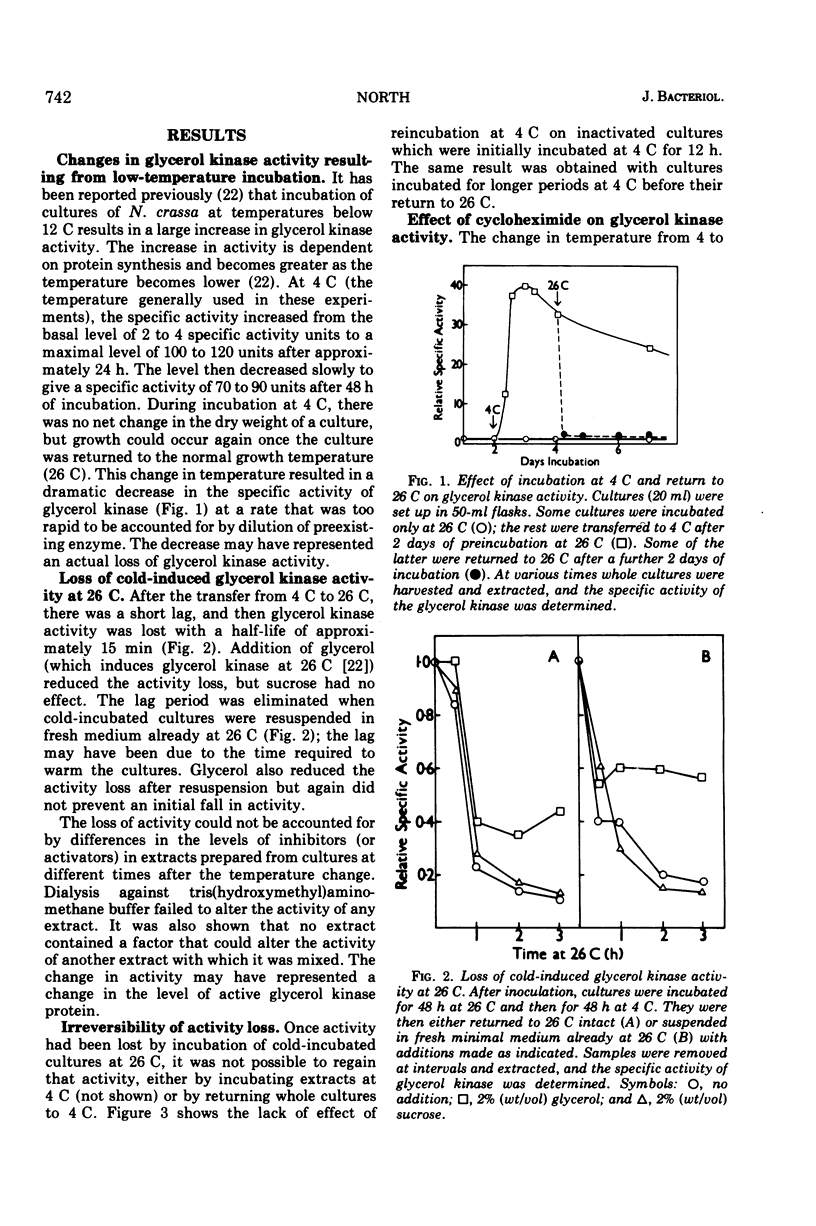
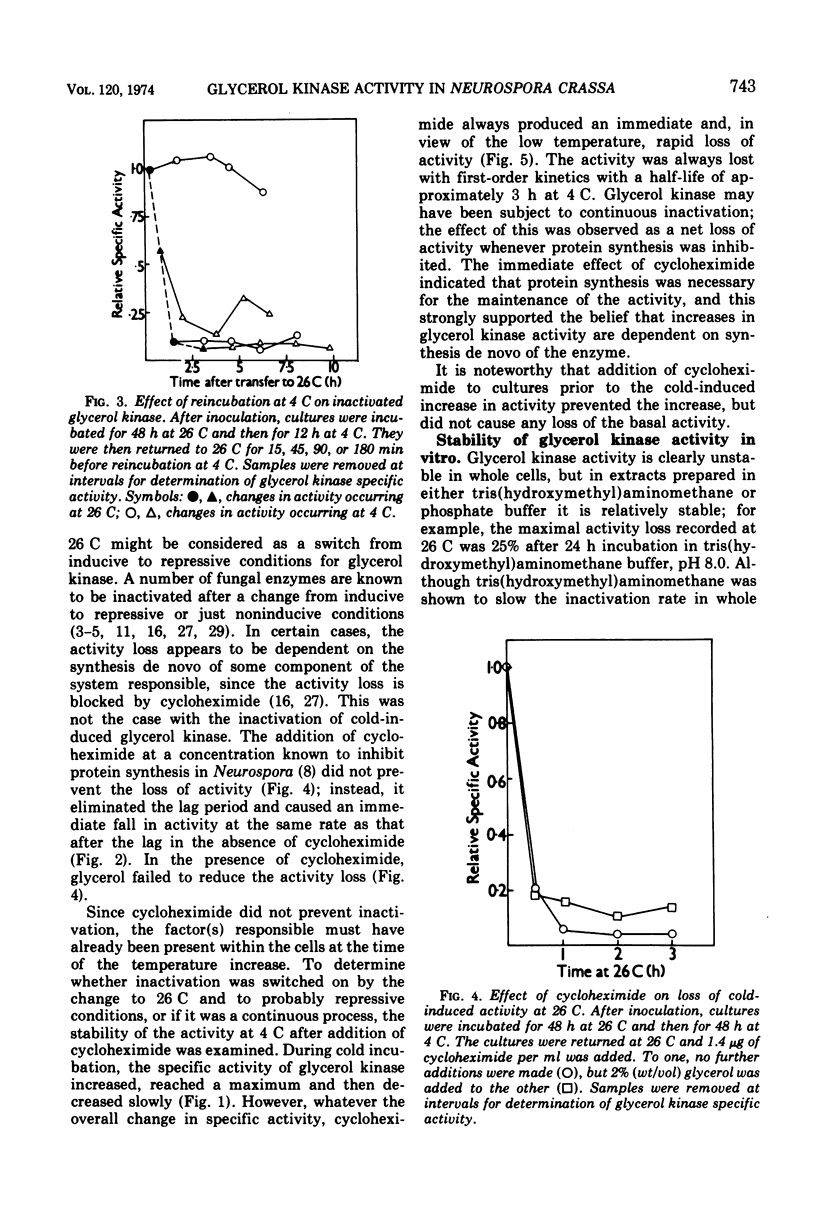
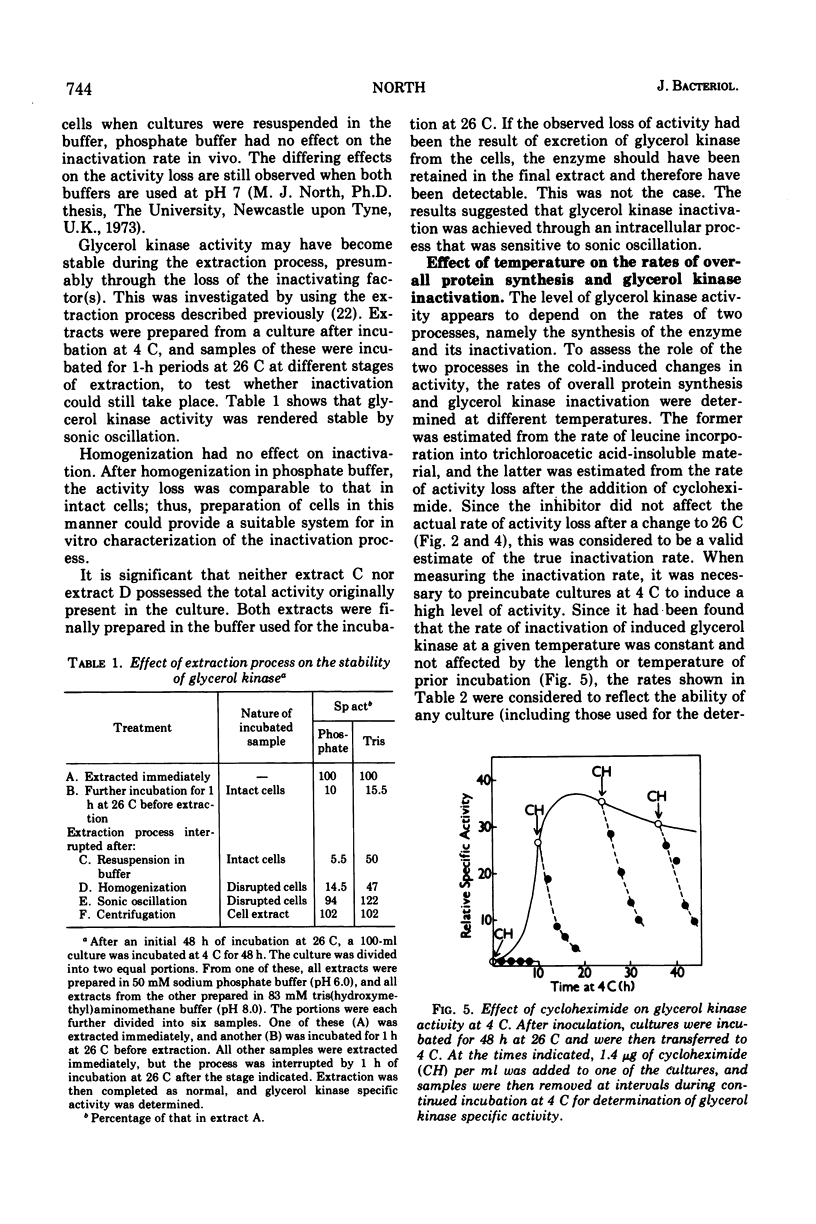
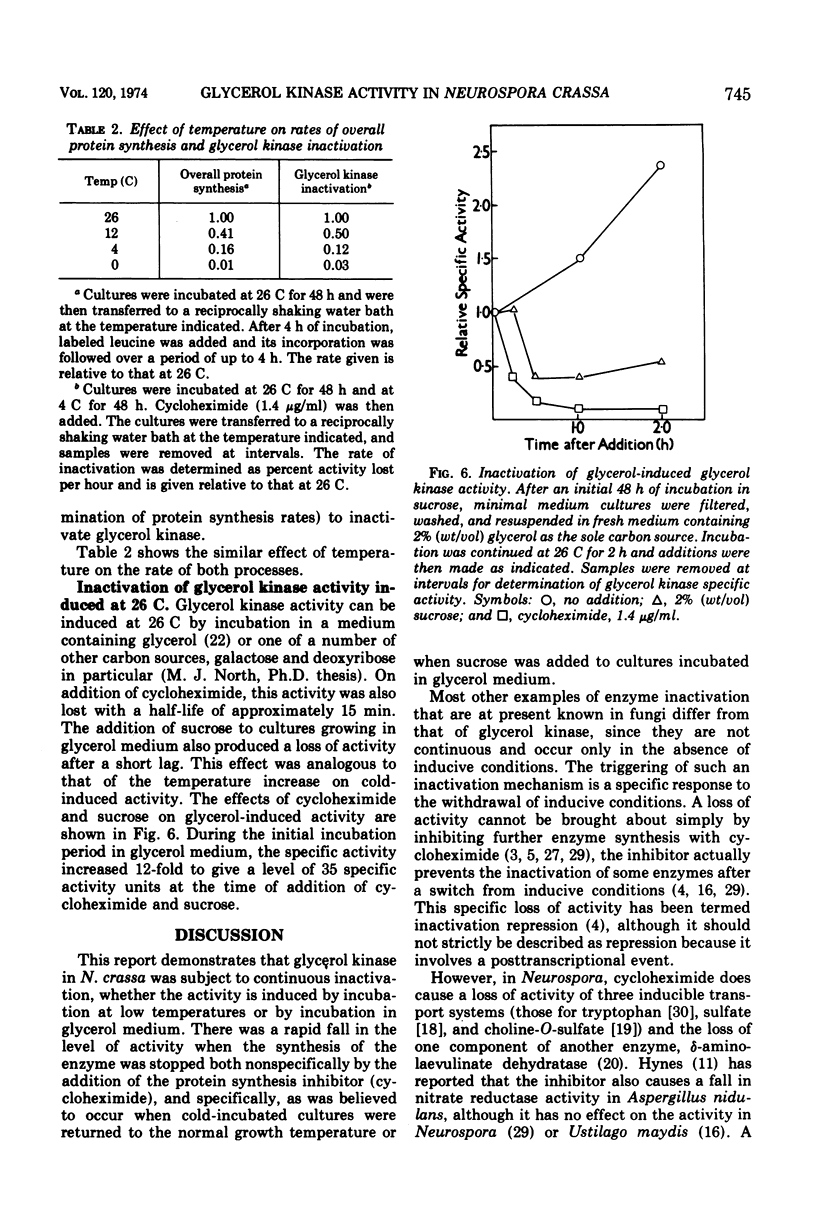
The glycerol kinase activity induced by incubation of Neurospora crassa at low temperatures was rapidly lost when cultures were returned to 26 C. After a short lag, the activity disappeared irreversibly with a half-life of approximately 15 min. The loss of activity was not due to a change in the level of an inhibitor or activator. Glycerol reduced the activity loss but did not completely prevent it, which was an effect that was dependent on protein synthesis. The cold-induced activity was also always lost on addition of cycloheximide at all temperatures tested (0 to 26 C), which indicated continuous inactivation, although cycloheximide did not affect the actual rate of activity loss at 26 C. The basal glycerol kinase activity was not sensitive to cycloheximide. The mechanism responsible for inactivation was destroyed by sonic oscillation. The process is not thought to play a role in the cold-induced increase in activity. Glycerol kinase activity induced at 26 C by glycerol was also lost on addition of cycloheximide and after addition of sucrose.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Cihak A., Lamar C., Jr, Pitot H. C. L-tryptophan inhibition of tyrosine aminotransferase degradation in rat liver in vivo. Arch Biochem Biophys. 1973 May;156(1):188–194. doi: 10.1016/0003-9861(73)90356-1. [DOI] [PubMed] [Google Scholar]
- Ferguson A. R., Sims A. P. The regulation of glutamine metabolism in Candida utilis: the inactivation of glutamine synthetase. J Gen Microbiol. 1974 Jan;80(1):173–185. doi: 10.1099/00221287-80-1-173. [DOI] [PubMed] [Google Scholar]
- Ferguson J. J., Jr, Boll M., Holzer H. Yeast malate dehydrogenase: enzyme inactivation in catabolite repression. Eur J Biochem. 1967 Mar;1(1):21–25. doi: 10.1007/978-3-662-25813-2_4. [DOI] [PubMed] [Google Scholar]
- Gancedo C. Inactivation of fructose-1,6-diphosphatase by glucose in yeast. J Bacteriol. 1971 Aug;107(2):401–405. doi: 10.1128/jb.107.2.401-405.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HOROWITZ N. H., FLING M., MACLEOD H., WATANABE Y. Structural and regulative genes controlling tyrosinase synthesis in Neurospora. Cold Spring Harb Symp Quant Biol. 1961;26:233–238. doi: 10.1101/sqb.1961.026.01.028. [DOI] [PubMed] [Google Scholar]
- Hayashi N., Yoda B., Kikuchi G. Mechanism of allylisopropylacetamide-induced increase of delta-aminolevulinate synthetase in liver mitochondria. IV. Accumulation of the enzyme in the soluble fraction of rat liver. Arch Biochem Biophys. 1969 Apr;131(1):83–91. doi: 10.1016/0003-9861(69)90107-6. [DOI] [PubMed] [Google Scholar]
- Holzer H., Katsunuma T., Schött E. G., Ferguson A. R., Hasilki A., Betz H. Studies on a tryptophan synthase inactivating system from yeast. Adv Enzyme Regul. 1973;11:53–60. doi: 10.1016/0065-2571(73)90008-3. [DOI] [PubMed] [Google Scholar]
- Horowitz N. H., Feldman H. M., Pall M. L. Derepression of tyrosinase synthesis in Neurospora by cycloheximide, actinomycin D, and puromycin. J Biol Chem. 1970 Jun 10;245(11):2784–2788. [PubMed] [Google Scholar]
- Horowitz N. H., Fling M., Feldman H. M., Pall M. L., Froehner S. C. Derepression of tyrosinase synthesis in Neurospora by amino acid analogs. Dev Biol. 1970 Feb;21(1):147–156. doi: 10.1016/0012-1606(70)90066-7. [DOI] [PubMed] [Google Scholar]
- Hynes M. J. The effect of lack of a carbon source on nitrate-reductase activity in Aspergillus nidulans. J Gen Microbiol. 1973 Nov;79(1):155–157. doi: 10.1099/00221287-79-1-155. [DOI] [PubMed] [Google Scholar]
- Jost J. P., Khairallah E. A., Pitot H. C. Studies on the induction and repression of enzymes in rat liver. V. Regulation of the rate of synthesis and degradation of serine dehydratase by dietary amino acids and glucose. J Biol Chem. 1968 Jun 10;243(11):3057–3066. [PubMed] [Google Scholar]
- Katunuma N., Kominami E., Kominami S., Kito K. Mode of action of specific inactivating enzymes for pyridoxal enzymes and NAD-dependent enzymes and their biological significance. Adv Enzyme Regul. 1972;10:289–306. doi: 10.1016/0065-2571(72)90019-2. [DOI] [PubMed] [Google Scholar]
- Kay J. E., Lindsay V. J., Cooke A. Ornithine decarboxylase in phytohaemagglutinin stimulated lymphocytes: Control of degradation rate by amino acids. FEBS Lett. 1972 Mar 15;21(2):123–126. doi: 10.1016/0014-5793(72)80118-2. [DOI] [PubMed] [Google Scholar]
- Kominami E., Kobayashi K., Kominami S., Katunuma N. Properties of a specific protease for pyridoxal enzymes and its biological role. J Biol Chem. 1972 Nov 10;247(21):6848–6855. [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Lewis C. M., Fincham J. R. Regulation of nitrate reductase in the basidiomycete Ustilago maydis. J Bacteriol. 1970 Jul;103(1):55–61. doi: 10.1128/jb.103.1.55-61.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marzluf G. A. Control of the synthesis, activity, and turnover of enzymes of sulfur metabolism in Neurospora crassa. Arch Biochem Biophys. 1972 Jun;150(2):714–724. doi: 10.1016/0003-9861(72)90090-2. [DOI] [PubMed] [Google Scholar]
- Marzluf G. A. Genetic and metabolic control of sulfate metabolism in Neurospora crassa: a specific permease for choline-O-sulfate. Biochem Genet. 1972 Dec;7(3):219–233. doi: 10.1007/BF00484820. [DOI] [PubMed] [Google Scholar]
- Muthukrishnan S., Malathi K., Padmanaban G. Delta-aminolaevulinate dehydratase, the regulatory enzyme of the haem-biosynthetic pathway in Neurospora crassa. Biochem J. 1972 Aug;129(1):31–37. doi: 10.1042/bj1290031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Newsholme E. A., Robinson J., Taylor K. A radiochemical enzymatic activity assay for glycerol kinase and hexokinase. Biochim Biophys Acta. 1967 Mar 15;132(2):338–346. doi: 10.1016/0005-2744(67)90153-2. [DOI] [PubMed] [Google Scholar]
- North M. J. Cold-induced increase of glycerol kinase in Neurospora crassa. FEBS Lett. 1973 Sep 1;35(1):67–70. doi: 10.1016/0014-5793(73)80578-2. [DOI] [PubMed] [Google Scholar]
- Russell D. H., Snyder S. H. Amine synthesis in regenerating rat liver: extremely rapid turnover of ornithine decarboxylase. Mol Pharmacol. 1969 May;5(3):253–262. [PubMed] [Google Scholar]
- SCHIMKE R. T., SWEENEY E. W., BERLIN C. M. THE ROLES OF SYNTHESIS AND DEGRADATION IN THE CONTROL OF RAT LIVER TRYPTOPHAN PYRROLASE. J Biol Chem. 1965 Jan;240:322–331. [PubMed] [Google Scholar]
- Saheki T., Holzer H. Comparisons of the tryptophan synthase inactivating enzymes with proteinases from yeast. Eur J Biochem. 1974 Mar 1;42(2):621–626. doi: 10.1111/j.1432-1033.1974.tb03377.x. [DOI] [PubMed] [Google Scholar]
- Schimke R. T. Control of enzyme levels in mammalian tissues. Adv Enzymol Relat Areas Mol Biol. 1973;37:135–187. doi: 10.1002/9780470122822.ch3. [DOI] [PubMed] [Google Scholar]
- Schneider R. P., Wiley W. R. Regulation of sugar transport in Neurospora crassa. J Bacteriol. 1971 May;106(2):487–492. doi: 10.1128/jb.106.2.487-492.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schött E. H., Holzer H. Purification and some properties of tryptophan synthase inactivase II from yeast. Eur J Biochem. 1974 Feb 15;42(1):61–66. doi: 10.1111/j.1432-1033.1974.tb03314.x. [DOI] [PubMed] [Google Scholar]
- Subramanian K. N., Sorger G. J. Regulation of nitrate reductase in Neurospora crassa: stability in vivo. J Bacteriol. 1972 May;110(2):538–546. doi: 10.1128/jb.110.2.538-546.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wiley W. R., Matchett W. H. Tryptophan transport in Neurospora crassa. II. Metabolic control. J Bacteriol. 1968 Mar;95(3):959–966. doi: 10.1128/jb.95.3.959-966.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]


