Abstract
Light above 400 nm had selective effects on Escherichia coli ML-308: several processes or enzymes were strongly inhibited, whereas others were relatively unaffected. There was a correlation between the inhibition of respiration and the inhibition of active uptake of glycine. However, phenylalanine uptake did not show such a correlation. The decrease in adenosine 5′-triphosphate level during the first few minutes of illumination resembled the inactivation kinetics of phenylalanine uptake. The results suggest that phenylalanine uptake may not depend greatly on oxidative energy and may depend on the adenosine 5′-triphosphate level. The results for glycine suggest either that its active uptake and respiration involve a common photosensitive component or alternately, that only the respiratory chain contains the photosensitive component, and that glycine uptake is coupled almost exclusively to respiration. The critical photochemical lesion does not involve d-lactate dehydrogenase, succinate dehydrogenase, or l-α-glycerophosphate dehydrogenase since their inactivation rate is markedly lower than that for respiration.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- ARRIGONI O., SINGER T. P. Limitations of the phenazine methosulphate assay for succinic and related dehydrogenases. Nature. 1962 Mar 31;193:1256–1258. doi: 10.1038/1931256a0. [DOI] [PubMed] [Google Scholar]
- Barnes E. M., Jr, Kaback H. R. Mechanisms of active transport in isolated membrane vesicles. I. The site of energy coupling between D-lactic dehydrogenase and beta-galactoside transport in Escherichia coli membrane vesicles. J Biol Chem. 1971 Sep 10;246(17):5518–5522. [PubMed] [Google Scholar]
- Barran L. R., Daoust J. Y., Labelle J. L., Martin W. G., Schneider H. Differential effects of visible light on active transport in E. coli. Biochem Biophys Res Commun. 1974 Jan 23;56(2):522–528. doi: 10.1016/0006-291x(74)90874-2. [DOI] [PubMed] [Google Scholar]
- Berger E. A. Different mechanisms of energy coupling for the active transport of proline and glutamine in Escherichia coli. Proc Natl Acad Sci U S A. 1973 May;70(5):1514–1518. doi: 10.1073/pnas.70.5.1514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CRANE R. K., LIPMANN F. The effect of arsenate on aerobic phosphorylation. J Biol Chem. 1953 Mar;201(1):235–243. [PubMed] [Google Scholar]
- Codd G. A. The photoinhibition of malate dehydrogenase. FEBS Lett. 1972 Feb 1;20(2):211–214. doi: 10.1016/0014-5793(72)80797-x. [DOI] [PubMed] [Google Scholar]
- Courtright J. B., Henning U. Malate dehydrogenase mutants in Escherichia coli K-12. J Bacteriol. 1970 Jun;102(3):722–728. doi: 10.1128/jb.102.3.722-728.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cox G. B., Snoswell A. M., Gibson F. The use of a ubiquinone-deficient mutant in the study of malate oxidation in Escherichia coli. Biochim Biophys Acta. 1968 Jan 15;153(1):1–12. doi: 10.1016/0005-2728(68)90140-0. [DOI] [PubMed] [Google Scholar]
- Epel B. L., Butler W. L. Inhibition of Respiration in Prototheca zopfii by Light. Plant Physiol. 1970 Jun;45(6):728–734. doi: 10.1104/pp.45.6.728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- FORREST W. W., WALKER D. J. SYNTHESIS OF RESERVE MATERIALS FOR ENDOGENOUS METABOLISM IN STREPTOCOCCUS FAECALIS. J Bacteriol. 1965 Jun;89:1448–1452. doi: 10.1128/jb.89.6.1448-1452.1965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Futai M. Membrane D-lactate dehydrogenase from Escherichia coli. Purification and properties. Biochemistry. 1973 Jun 19;12(13):2468–2474. doi: 10.1021/bi00737a016. [DOI] [PubMed] [Google Scholar]
- Hirata H., Asano A., Brodie A. F. Respiration dependent transport of proline by electron transport particles from mycobacterium phlei. Biochem Biophys Res Commun. 1971 Jul 16;44(2):368–374. doi: 10.1016/0006-291x(71)90609-7. [DOI] [PubMed] [Google Scholar]
- Hong J. S., Kaback H. R. Mutants of Salmonella typhimurium and Escherichia coli pleiotropically defective in active transport. Proc Natl Acad Sci U S A. 1972 Nov;69(11):3336–3340. doi: 10.1073/pnas.69.11.3336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KASHKET E. R., BRODIE A. F. Oxidative phosphorylation in fractionated bacterial systems. X. Different roles for the natural quinones of Escherichia coli W in oxidative metabolism. J Biol Chem. 1963 Jul;238:2564–2570. [PubMed] [Google Scholar]
- Kaback H. R. Transport across isolated bacterial cytoplasmic membranes. Biochim Biophys Acta. 1972 Aug 4;265(3):367–416. doi: 10.1016/0304-4157(72)90014-7. [DOI] [PubMed] [Google Scholar]
- Klein W. L., Boyer P. D. Energization of active transport by Escherichia coli. J Biol Chem. 1972 Nov 25;247(22):7257–7265. [PubMed] [Google Scholar]
- Kohn L. D., Kaback H. R. Mechanisms of active transport in isolated bacterial membrane vesicles. XV. Purification and properties of the membrane-bound D-lactate dehydrogenase from Escherichia coli. J Biol Chem. 1973 Oct 25;248(20):7012–7017. [PubMed] [Google Scholar]
- LIN E. C., KOCH J. P., CHUSED T. M., JORGENSEN S. E. Utilization of L-alpha-glycerophosphate by Escherichia coli without hydrolysis. Proc Natl Acad Sci U S A. 1962 Dec 15;48:2145–2150. doi: 10.1073/pnas.48.12.2145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- MacLeod R. A., Thurman P., Rogers H. J. Comparative transport activity of intact cells, membrane vesicles, and mesosomes of Bacillus licheniformis. J Bacteriol. 1973 Jan;113(1):329–340. doi: 10.1128/jb.113.1.329-340.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ne'eman Z., Kahane I., Razin S. Characterization of the mycoplasma membrane proteins. II. Solubilization and enzymic activities of Acholeplasma laidlawii membrane proteins. Biochim Biophys Acta. 1971 Oct 12;249(1):169–176. doi: 10.1016/0005-2736(71)90093-9. [DOI] [PubMed] [Google Scholar]
- Ninnemann H. Photoinhibition of isolated complexes I, II, and 3 of beef heart mitochondria. FEBS Lett. 1974 Mar 1;39(3):353–358. doi: 10.1016/0014-5793(74)80148-1. [DOI] [PubMed] [Google Scholar]
- Ninnemann H. Photoinhibition of respiration in bacteria and the cyanophycea Vitreoscilla stercoraria. FEBS Lett. 1972 Oct 15;27(1):179–180. doi: 10.1016/0014-5793(72)80436-8. [DOI] [PubMed] [Google Scholar]
- Roisin M. P., Kepes A. The membrane ATPase of Escherichia coli. I. Ion dependence and ATP-ADP exchange reaction. Biochim Biophys Acta. 1972 Sep 20;275(3):333–346. doi: 10.1016/0005-2728(72)90214-9. [DOI] [PubMed] [Google Scholar]
- SCOTT D. B., COHEN S. S. The oxidative pathway of carbohydrate metabolism in Escherichia coli. 1. The isolation and properties of glucose 6-phosphate dehydrogenase and 6-phosphogluconate dehydrogenase. Biochem J. 1953 Aug;55(1):23–33. doi: 10.1042/bj0550023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sprott G. D., MacLeod R. A. Na + -dependent amino acid transport in isolated membrane vesicles of a marine pseudomonad energized by electron donors. Biochem Biophys Res Commun. 1972 May 26;47(4):838–845. doi: 10.1016/0006-291x(72)90569-4. [DOI] [PubMed] [Google Scholar]
- Weber K., Osborn M. The reliability of molecular weight determinations by dodecyl sulfate-polyacrylamide gel electrophoresis. J Biol Chem. 1969 Aug 25;244(16):4406–4412. [PubMed] [Google Scholar]
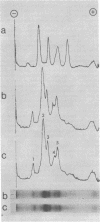
- Weiner J. H., Heppel L. A. Purification of the membrane-bound and pyridine nucleotide-independent L-glycerol 3-phosphate dehydrogenase from Escherichia coli. Biochem Biophys Res Commun. 1972 Jun 28;47(6):1360–1365. doi: 10.1016/0006-291x(72)90222-7. [DOI] [PubMed] [Google Scholar]