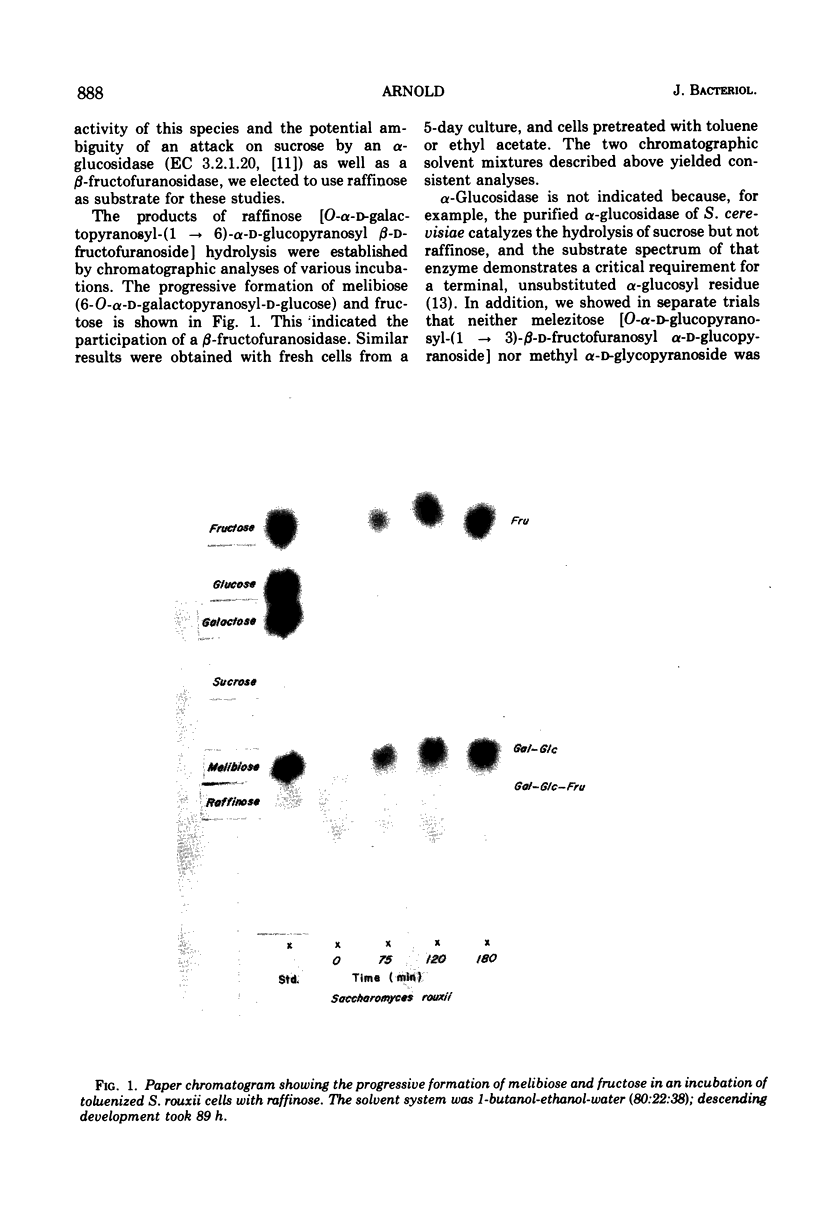
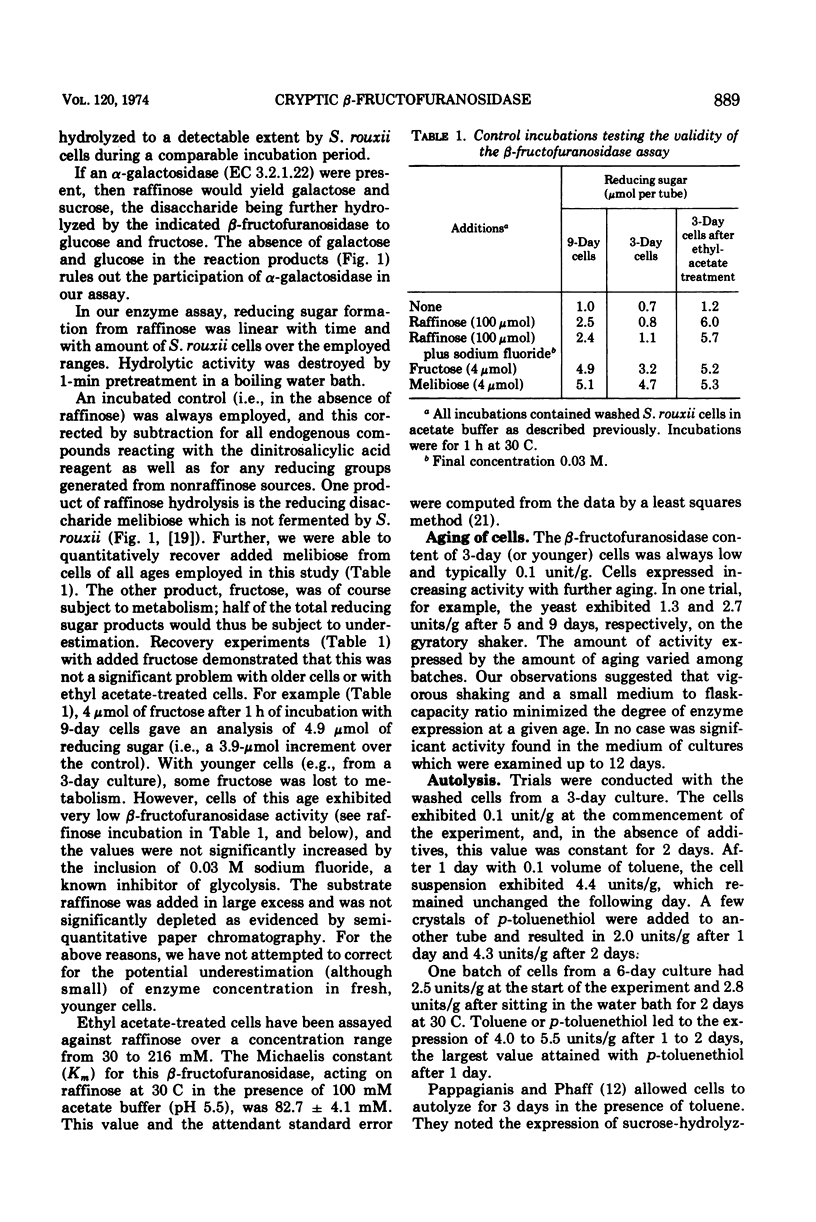
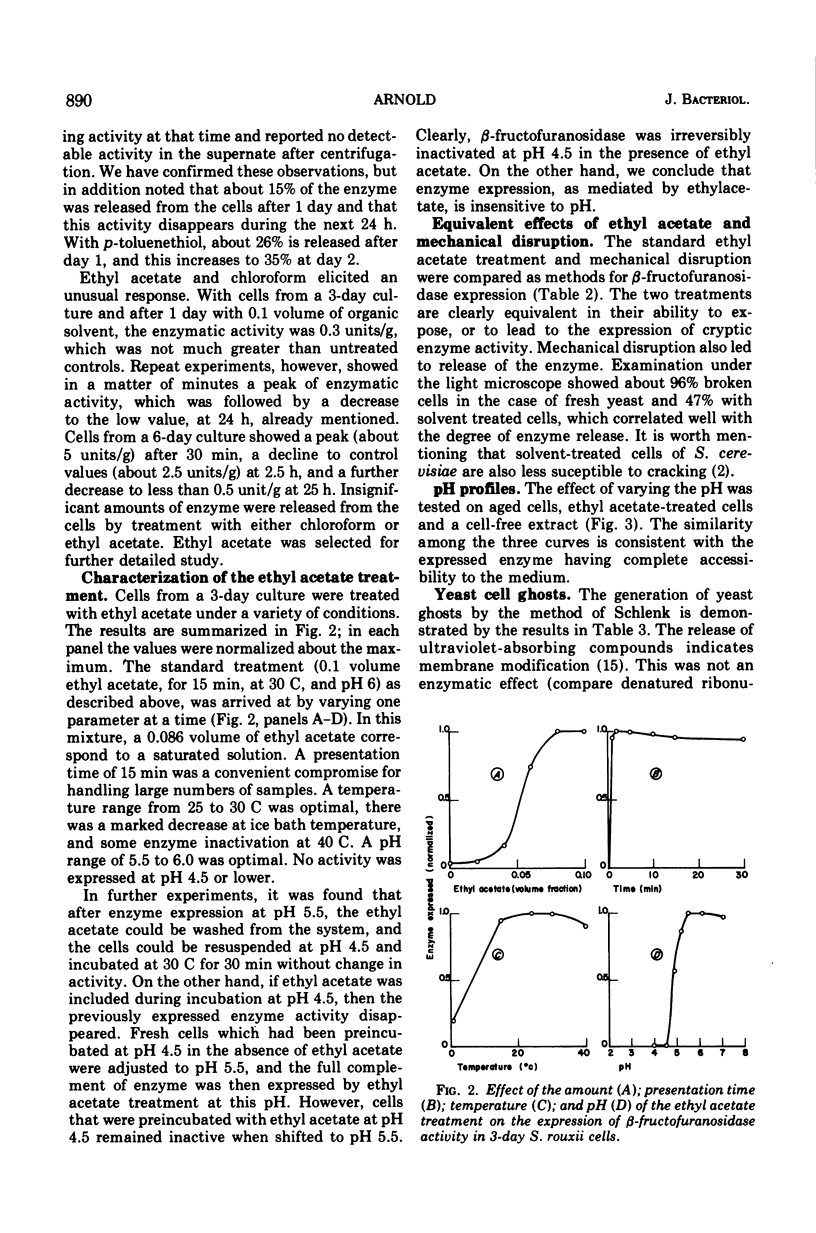
Abstract
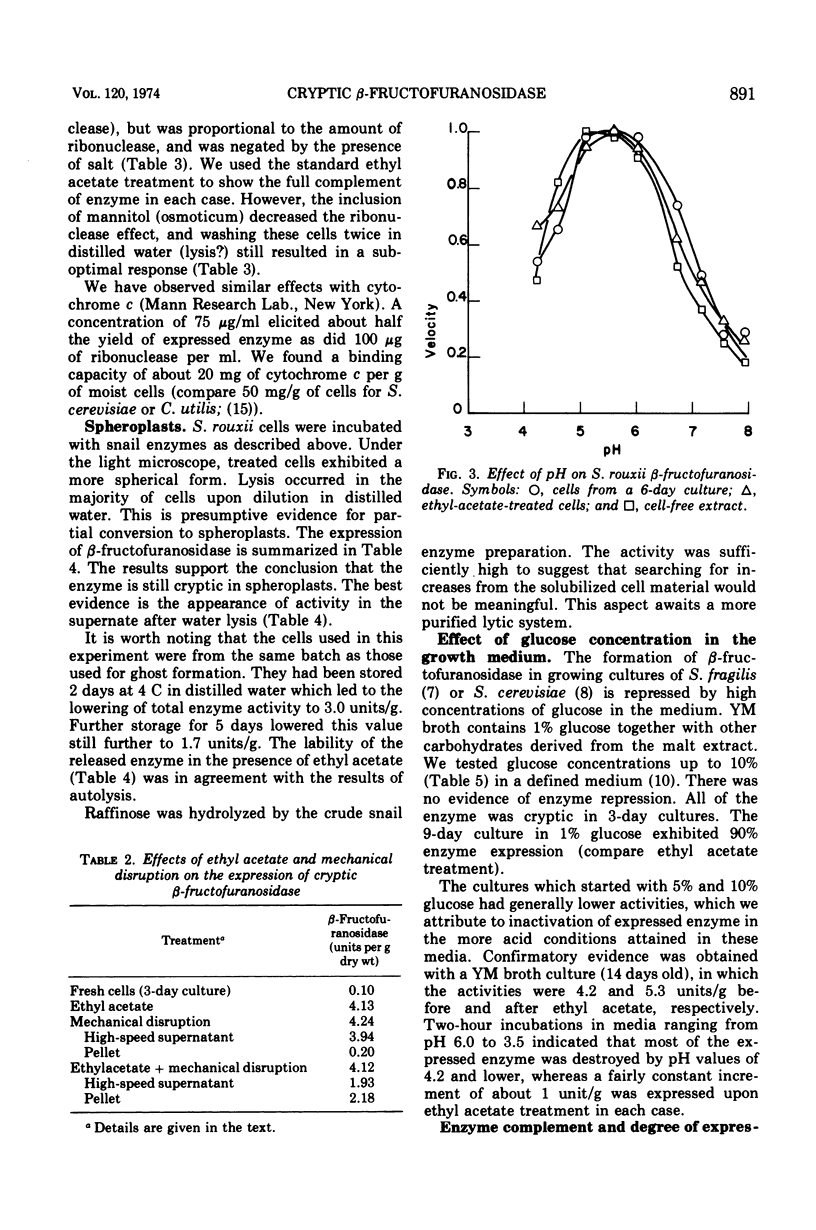
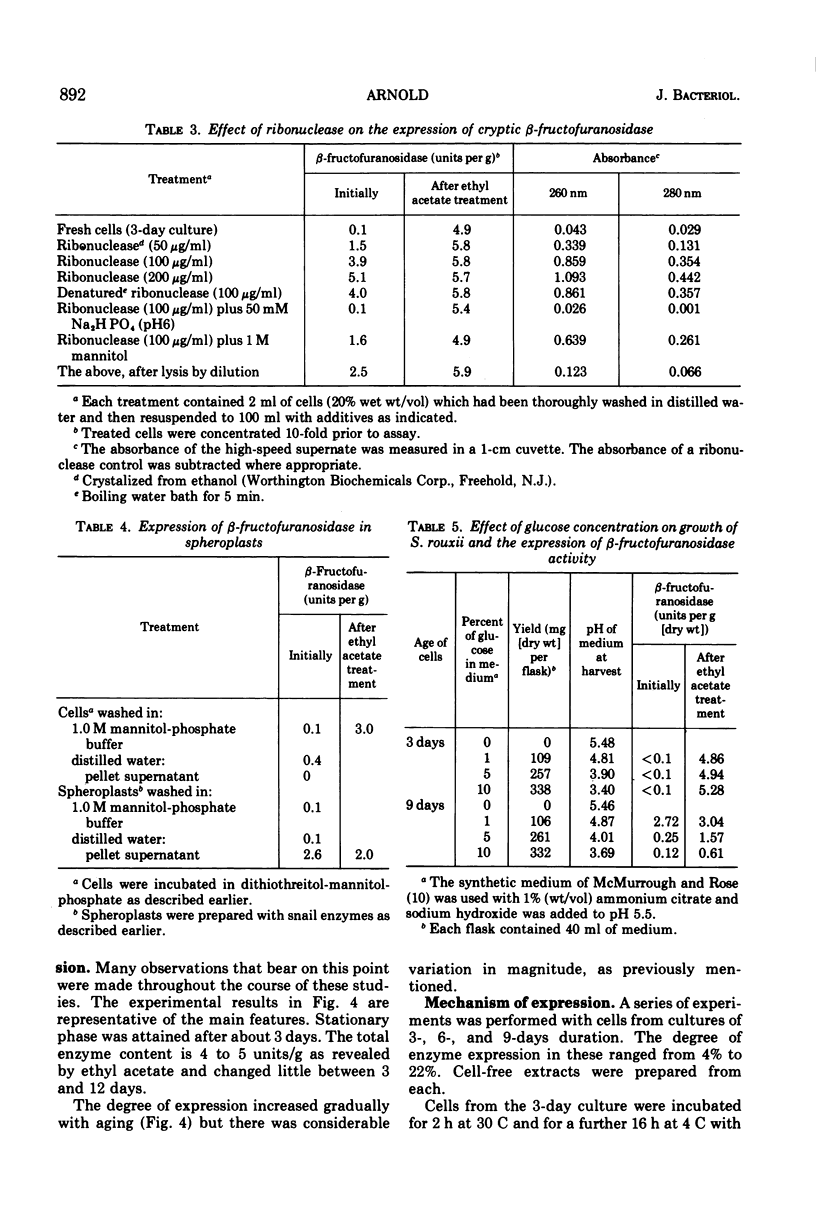
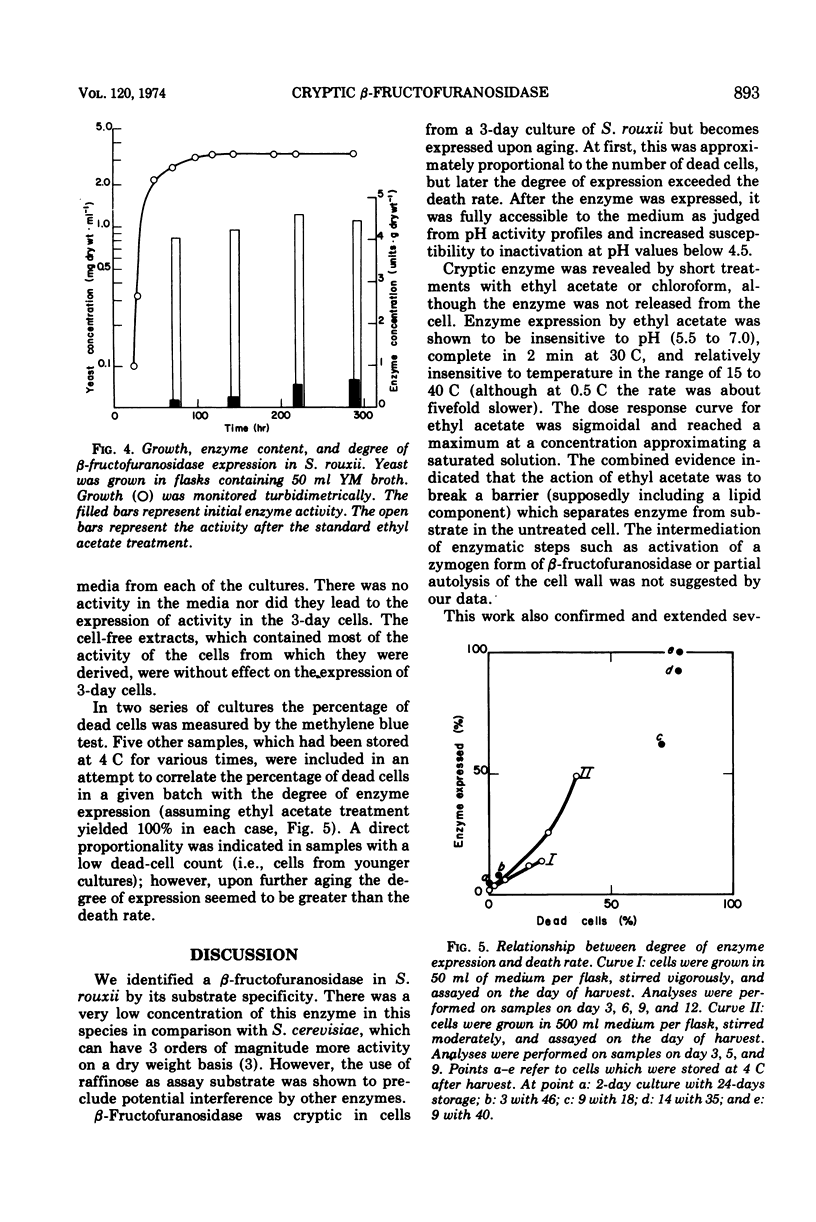
Raffinose hydrolysis was studied in Saccharomyces rouxii. The responsible enzyme was identified as a β-fructofuranosidase (EC 3.2.1.26), which has a pH optimum of 5.5 and a Km of 83 mM for raffinose. This enzyme was cryptic in cells from a 3-day culture. A 2-min treatment with 0.1 volume of ethyl acetate in sodium acetate buffer (pH 6) gave complete expression of the enzyme, which was still retained by the cell. Ghosts were prepared by modifying membrane structure with small basic proteins in distilled water, and after washing they showed the full complement of enzymatic activity. The enzyme remained cryptic in osmotically protected spheroplasts; however, after lysis (by dilution) release, as well as expression, was effected. Mechanical disruption of fresh cells revealed and released all of the enzyme. Cells in early stationary phase had all of their β-fructofuranosidase in a cryptic state. Aging yielded fractional expression of activity; initially this was proportional to cell death, but later the degree of expression exceeded the death rate. Media from aged cultures or cell-free extracts of aged cells were not effective in revealing the cryptic enzyme of younger cells. S. rouxii β-fructofuranosidase has a different autolytic-release pattern from its counterpart in S. cerevisiae. Also, high concentrations of glucose do not repress the S. rouxii enzyme. The β-fructofuranosidase in young cells of S. rouxii must be enclosed by the protoplasmic membrane or a special vesicular structure. This system was compared with other Saccharomyces species in connection with the translocation of enzymes across the protoplasmic membrane.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Alper R. E., Dainko J. L., Schlenk F. Properties of yeast cell ghosts obtained by ribonuclease action. J Bacteriol. 1967 Feb;93(2):759–765. doi: 10.1128/jb.93.2.759-765.1967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arnold W. N. Location of acid phosphatase and -fructofuranosidase within yeast cell envelopes. J Bacteriol. 1972 Dec;112(3):1346–1352. doi: 10.1128/jb.112.3.1346-1352.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arnold W. N. The structure of the yeast cell wall. Solubilization of a marker enzyme, -fructofuranosidase, by the autolytic enzyme system. J Biol Chem. 1972 Feb 25;247(4):1161–1169. [PubMed] [Google Scholar]
- Arnold W. N. p-Toluenethiol as an initiator of autolysis in bakers' yeast. J Bacteriol. 1972 Feb;109(2):949–951. doi: 10.1128/jb.109.2.949-951.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BURGER M., BACON E. E., BACON J. S. Some observations on the form and location of invertase in the yeast cell. Biochem J. 1961 Mar;78:504–511. doi: 10.1042/bj0780504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DAVIES R. Enzyme formation in Saccharomyces fragilis. I. Invertase and raffinase. Biochem J. 1953 Oct;55(3):484–497. doi: 10.1042/bj0550484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DODYK F., ROTHSTEIN A. FACTORS INFLUENCING THE APPEARANCE OF INVERTASE IN SACCHAROMYCES CEREVISIAE. Arch Biochem Biophys. 1964 Mar;104:478–486. doi: 10.1016/0003-9861(64)90492-8. [DOI] [PubMed] [Google Scholar]
- Kidby D. K., Davies R. Thiol induced release of invertase from cell walls of Saccharomyces fragilis. Biochim Biophys Acta. 1970 Feb 24;201(2):261–266. doi: 10.1016/0304-4165(70)90300-4. [DOI] [PubMed] [Google Scholar]
- McMurrough I., Rose A. H. Effect of growth rate and substrate limitation on the composition and structure of the cell wall of Saccharomyces cerevisiae. Biochem J. 1967 Oct;105(1):189–203. doi: 10.1042/bj1050189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- PAPPAGIANIS D., PHAFF H. J. Delayed fermentation of sucrose by certain haploid species of Saccharomyces. Antonie Van Leeuwenhoek. 1956;22(4):353–370. doi: 10.1007/BF02538349. [DOI] [PubMed] [Google Scholar]
- Schlenk F., Zydek-Cwick C. R. Enzymatic activity of yeast cell ghosts produced by protein action on the membranes. Arch Biochem Biophys. 1970 May;138(1):220–225. doi: 10.1016/0003-9861(70)90301-2. [DOI] [PubMed] [Google Scholar]
- Schwencke J., Farías G., Rojas M. The release of extracellular enzymes from yeast by "osmotic shock". Eur J Biochem. 1971 Jul 15;21(1):137–143. doi: 10.1111/j.1432-1033.1971.tb01449.x. [DOI] [PubMed] [Google Scholar]
- TREVELYAN W. E., PROCTER D. P., HARRISON J. S. Detection of sugars on paper chromatograms. Nature. 1950 Sep 9;166(4219):444–445. doi: 10.1038/166444b0. [DOI] [PubMed] [Google Scholar]
- WICKERHAM L. J. Evidence of the production of extracellular invertase by certain strains of yeasts. Arch Biochem Biophys. 1958 Aug;76(2):439–448. doi: 10.1016/0003-9861(58)90169-3. [DOI] [PubMed] [Google Scholar]
- WILKINSON G. N. Statistical estimations in enzyme kinetics. Biochem J. 1961 Aug;80:324–332. doi: 10.1042/bj0800324. [DOI] [PMC free article] [PubMed] [Google Scholar]