Abstract
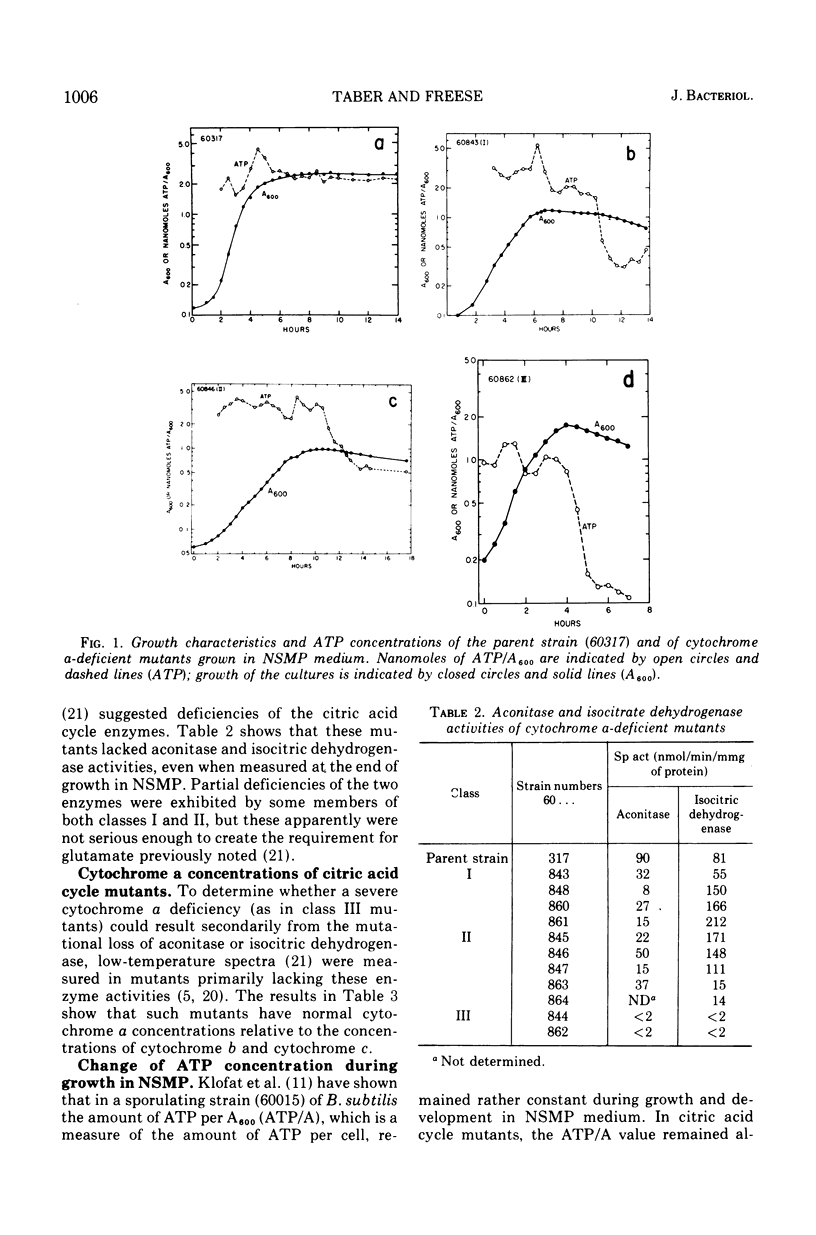
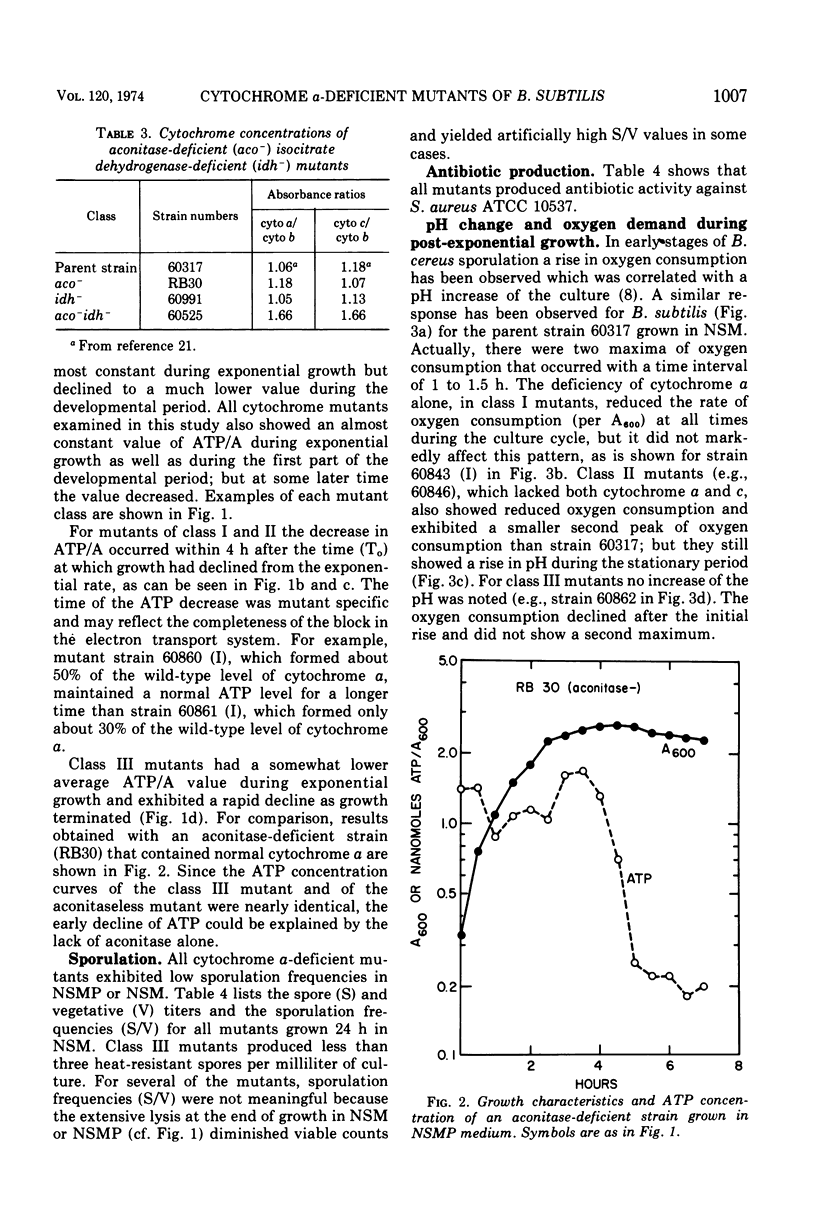
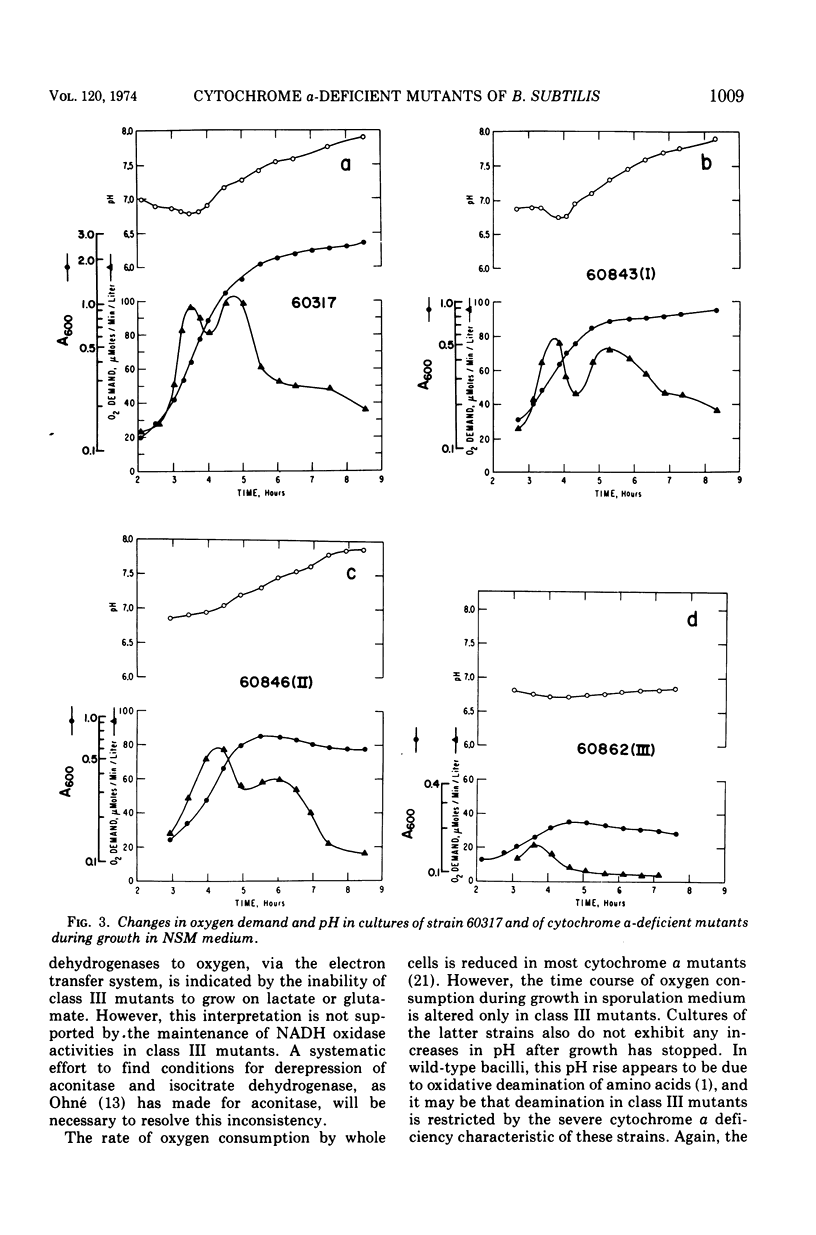
Three classes of cytochrome a-deficient mutants of Bacillus subtilis have been found to be asporogenic or oligosporogenic. All three classes showed declines in adenosine 5′-triphosphate (ATP) concentrations during early sporulation, at a time when ATP levels in wild-type strains are constant. Class III mutants were found to be deficient in aconitase and isocitric dehydrogenase, and showed reduced maximum growth in nutrient sporulation medium. These mutants also suffered the most rapid decline in ATP concentration in early sporulation, and exhibited neither the biphasic oxygen consumption curve nor the increase in pH normally observed at the end of logarithmic growth in nutrient sporulation medium. Nicotinamide adenine dinucleotide oxidase activities of purified membrane preparations were approximately normal for mutants in all classes, except for two of the class II mutants and one class III mutant. Neither cytochrome a nor cytochrome c appears to be an obligatory intermediate in cyanide-sensitive nicotinamide adenine dinucleotide oxidation in B. subtilis.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Broberg P. L., Smith L. The cytochrome system of Bacillus megaterium KM. The presence and some properties of two CO-binding cytochromes. Biochim Biophys Acta. 1967 May 9;131(3):479–489. doi: 10.1016/0005-2728(67)90007-2. [DOI] [PubMed] [Google Scholar]
- CHAIX P., PETIT J. F. Etude de différents spectres cytochromiques de Bacillus subtilis. Biochim Biophys Acta. 1956 Oct;22(1):66–71. doi: 10.1016/0006-3002(56)90224-4. [DOI] [PubMed] [Google Scholar]
- Fortnagel P., Freese E. Analysis of sporulation mutants. II. Mutants blocked in the citric acid cycle. J Bacteriol. 1968 Apr;95(4):1431–1438. doi: 10.1128/jb.95.4.1431-1438.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fortnagel P. The regulation of aconitase and isocitrate dehydrogenase in sporulation mutants of Bacillus subtilis. Biochim Biophys Acta. 1970 Nov 24;222(2):290–298. doi: 10.1016/0304-4165(70)90116-9. [DOI] [PubMed] [Google Scholar]
- Freese E. B. Unusual membranous structures in cytochrome a-deficient mutants of Bacillus subtilis. J Gen Microbiol. 1973 Mar;75(1):187–190. doi: 10.1099/00221287-75-1-187. [DOI] [PubMed] [Google Scholar]
- Hanson R. S., Cox D. P. Effect of different nutritional conditions on the synthesis of tricarboxylic acid cycle enzymes. J Bacteriol. 1967 Jun;93(6):1777–1787. doi: 10.1128/jb.93.6.1777-1787.1967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klofat W., Picciolo G., Chappelle E. W., Freese E. Production of adenosine triphosphate in normal cells and sporulation mutants of Bacillus subtilis. J Biol Chem. 1969 Jun 25;244(12):3270–3276. [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Ohné M. Regulation of aconitase synthesis in Bacillus subtilis: induction, feedback repression, and catabolite repression. J Bacteriol. 1974 Mar;117(3):1295–1305. doi: 10.1128/jb.117.3.1295-1305.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohné M., Rutberg B., Hoch J. A. Genetic and biochemical characterization of mutants of Bacillus subtilis defective in succinate dehydrogenase. J Bacteriol. 1973 Sep;115(3):738–745. doi: 10.1128/jb.115.3.738-745.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- RACKER E. Spectrophotometric measurements of the enzymatic formation of fumaric and cis-aconitic acids. Biochim Biophys Acta. 1950 Jan;4(1-3):211–214. doi: 10.1016/0006-3002(50)90026-6. [DOI] [PubMed] [Google Scholar]
- REILLY C., SHERMAN R. GLUCOSE REPRESSION OF CYTOCHROME A-SYNTHESIS IN CYTOCHROME-DEFICIENT MUTANTS OF YEAST. Biochim Biophys Acta. 1965 Apr 19;95:640–651. doi: 10.1016/0005-2787(65)90518-6. [DOI] [PubMed] [Google Scholar]
- Spizizen J. TRANSFORMATION OF BIOCHEMICALLY DEFICIENT STRAINS OF BACILLUS SUBTILIS BY DEOXYRIBONUCLEATE. Proc Natl Acad Sci U S A. 1958 Oct 15;44(10):1072–1078. doi: 10.1073/pnas.44.10.1072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Staal S. P., Hoch J. A. Conditional dihydrostreptomycin resistance in Bacillus subtilis. J Bacteriol. 1972 Apr;110(1):202–207. doi: 10.1128/jb.110.1.202-207.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taber H. Isolation and properties of cytochrome a deficient mutants of Bacillus subtilis. J Gen Microbiol. 1974 Apr;81(2):435–444. doi: 10.1099/00221287-81-2-435. [DOI] [PubMed] [Google Scholar]
- Tochikubo K. Changes in terminal respiratory pathways of Bacillus subtilis during germination, outgrowth and vegetative growth. J Bacteriol. 1971 Nov;108(2):652–661. doi: 10.1128/jb.108.2.652-661.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]


