Abstract
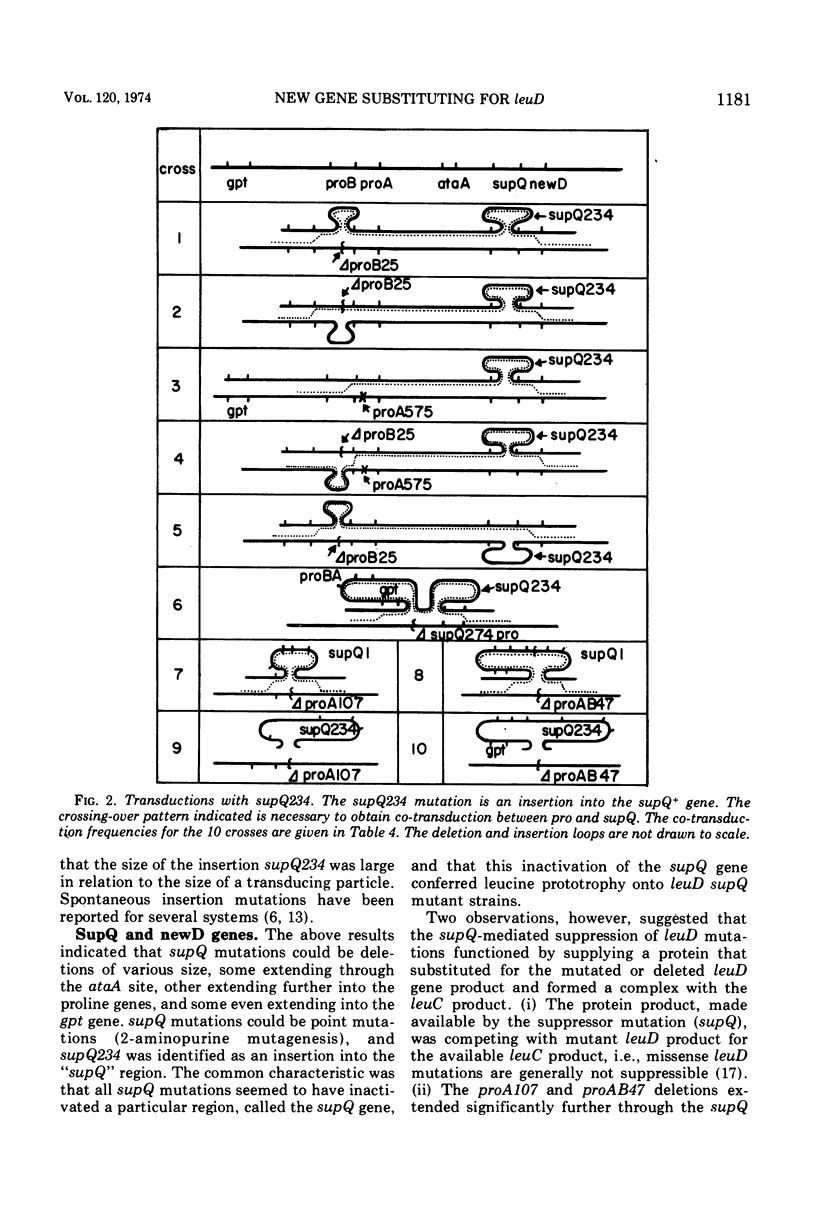
The second specific enzyme in the biosynthesis of leucine, α-isopropylmalate isomerase, is coded for by two genes, leuC and leuD. Leucine auxotrophs carrying mutations in the leuD gene (including deletions of the entire leuD gene) revert to leucine prototrophy by secondary mutations at the locus supQ, which is located in the proline region of the chromosome. The mechanism of the supQ function is explained by the following model. The supQ gene and an additional gene, newD, code for two different subunits of a multimeric enzyme, whose normal function is yet to be determined. The newD gene protein is able, without genetic alterations, to form an active complex with the leuC protein, thus replacing the nonfunctional or missing leuD protein and restoring leucine prototrophy. The newD protein has, however, a higher affinity for the supQ protein than for the leuC protein; therefore, mutations in the supQ gene are needed to make sufficient amounts of the newD protein available. The following gene order has been established: gpt-proB-proA-ataA-supQ-newD. Different supQ mutations have been identified, i.e., insertion in the supQ gene, point mutations, and deletions of various extent. Some deletions remove the P22 phage attachment site ataA. Other supQ deletions are simultaneously Pro−, because they extend into the proA or proA and proB genes; some extend even further, i.e., into the gpt gene (guanine phosphoribosyl transferase). Mutations in the newD gene caused renewed leucine auxotrophy in leuD supQ mutant strains. One newD mutation causes a temperature-sensitive Leu+ phenotype. Alternate models for the supQ-newD interactions are discussed.
Full text
PDF








Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- AMES B. N., HARTMAN P. E., JACOB F. Chromosomal alterations affecting the regulation of histidine biosynthetic enzymes in Salmonella. J Mol Biol. 1963 Jul;7:23–42. doi: 10.1016/s0022-2836(63)80016-9. [DOI] [PubMed] [Google Scholar]
- BECKWITH J. R. A DELETION ANALYSIS OF THE LAC OPERATOR REGION IN ESCHERICHIA COLI. J Mol Biol. 1964 Mar;8:427–430. doi: 10.1016/s0022-2836(64)80206-0. [DOI] [PubMed] [Google Scholar]
- Bagdian G., Mäkelä P. H. Antigenic conversion by phage P27. I. Mapping of the prophage attachment site on the Salmonella chromosome. Virology. 1971 Feb;43(2):403–411. doi: 10.1016/0042-6822(71)90312-6. [DOI] [PubMed] [Google Scholar]
- Berkowitz D., Hushon J. M., Whitfield H. J., Jr, Roth J., Ames B. N. Procedure for identifying nonsense mutations. J Bacteriol. 1968 Jul;96(1):215–220. doi: 10.1128/jb.96.1.215-220.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Campbell J. H., Lengyel J. A., Langridge J. Evolution of a second gene for beta-galactosidase in Escherichia coli. Proc Natl Acad Sci U S A. 1973 Jun;70(6):1841–1845. doi: 10.1073/pnas.70.6.1841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fiandt M., Szybalski W., Malamy M. H. Polar mutations in lac, gal and phage lambda consist of a few IS-DNA sequences inserted with either orientation. Mol Gen Genet. 1972;119(3):223–231. doi: 10.1007/BF00333860. [DOI] [PubMed] [Google Scholar]
- GROSS S. R., BURNS R. O., UMBARGER H. E. THE BIOSYNTHESIS OF LEUCINE. II. THE ENZYMIC ISOMERIZATION OF BETA-CARBOXY-BETA-HYDROXYISOCAPROATE AND ALPHA-HYDROXY-BETA-CARBOXYISOCAPROATE. Biochemistry. 1963 Sep-Oct;2:1046–1052. doi: 10.1021/bi00905a023. [DOI] [PubMed] [Google Scholar]
- GROSS S. R. On the mechanism of complementation at the LEU-2 locus of Neurospora. Proc Natl Acad Sci U S A. 1962 Jun 15;48:922–930. doi: 10.1073/pnas.48.6.922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gots J. S., Benson C. E., Shumas S. R. Genetic separation of hypoxanthine and guanine-xanthine phosphoribosyltransferase activities by deletion mutations in Salmonella typhimurium. J Bacteriol. 1972 Nov;112(2):910–916. doi: 10.1128/jb.112.2.910-916.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hall B. G., Hartl D. L. Regulation of newly evolved enzymes. I. Selection of a novel lactase regulated by lactose in Escherichia coli. Genetics. 1974 Mar;76(3):391–400. doi: 10.1093/genetics/76.3.391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hartl D. L., Hall B. G. Second naturally occurring beta-galactosidase in E. coli. Nature. 1974 Mar 8;248(5444):152–153. doi: 10.1038/248152a0. [DOI] [PubMed] [Google Scholar]
- Hegeman G. D., Rosenberg S. L. The evolution of bacterial enzyme systems. Annu Rev Microbiol. 1970;24:429–462. doi: 10.1146/annurev.mi.24.100170.002241. [DOI] [PubMed] [Google Scholar]
- Hirsch H. J., Starlinger P., Brachet P. Two kinds of insertions in bacterial genes. Mol Gen Genet. 1972;119(3):191–206. doi: 10.1007/BF00333858. [DOI] [PubMed] [Google Scholar]
- Itikawa H., Demerec M. Salmonella typhimurium proline mutants. J Bacteriol. 1968 Mar;95(3):1189–1190. doi: 10.1128/jb.95.3.1189-1190.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kemper J. Evolution of a new gene substituting for the leuD gene of Salmonella typhimurium: characterization of supQ mutations. J Bacteriol. 1974 Sep;119(3):937–951. doi: 10.1128/jb.119.3.937-951.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kemper J. Gene order and co-transduction in the leu-ara-fol-pyrA region of the Salmonella typhimurium linkage map. J Bacteriol. 1974 Jan;117(1):94–99. doi: 10.1128/jb.117.1.94-99.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuo T. T., Stocker B. A. Suppression of proline requirement of proA and proAB deletion mutants in Salmonella typhimurium by mutation to arginine requirement. J Bacteriol. 1969 May;98(2):593–598. doi: 10.1128/jb.98.2.593-598.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LEVINE M. Mutations in the temperate phage P22 and lysogeny in Salmonella. Virology. 1957 Feb;3(1):22–41. doi: 10.1016/0042-6822(57)90021-1. [DOI] [PubMed] [Google Scholar]
- MARGOLIN P. Genetic fine structure of the leucine operon in Salmonella. Genetics. 1963 Mar;48:441–457. doi: 10.1093/genetics/48.3.441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Margolin P., Bauerle R. H. Determinants for regulation and initiation of expression of tryptophan genes. Cold Spring Harb Symp Quant Biol. 1966;31:311–320. doi: 10.1101/sqb.1966.031.01.041. [DOI] [PubMed] [Google Scholar]
- Skarstedt M. T., Greer S. B. Threonine synthetase of Bacillus subtilis. The nature of an associated dehydratase activity. J Biol Chem. 1973 Feb 10;248(3):1032–1044. [PubMed] [Google Scholar]
- Smith H. O., Levine M. A phage P22 gene controlling integration of prophage. Virology. 1967 Feb;31(2):207–216. doi: 10.1016/0042-6822(67)90164-x. [DOI] [PubMed] [Google Scholar]
- Smith H. O., Levine M. Gene order in prophage P22. Virology. 1965 Oct;27(2):229–231. doi: 10.1016/0042-6822(65)90166-2. [DOI] [PubMed] [Google Scholar]
- Somers J. M., Amzallag A., Middleton R. B. Genetic fine structure of the leucine operon of Escherichia coli K-12. J Bacteriol. 1973 Mar;113(3):1268–1272. doi: 10.1128/jb.113.3.1268-1272.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- St Pierre M. L. Mutations creating a new initiation point for expression of the histidine operon in Salmonella typhimurium. J Mol Biol. 1968 Jul 14;35(1):71–82. doi: 10.1016/s0022-2836(68)80037-3. [DOI] [PubMed] [Google Scholar]
- TESSMAN I. The induction of large deletions by nitrous acid. J Mol Biol. 1962 Oct;5:442–445. doi: 10.1016/s0022-2836(62)80033-3. [DOI] [PubMed] [Google Scholar]
- Vapnek D., Greer S. Minor threonine dehydratase encoded within the threonine synthetic region of Bacillus subtilis. J Bacteriol. 1971 Jun;106(3):983–993. doi: 10.1128/jb.106.3.983-993.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wuesthoff G., Bauerle R. H. Mutations creating internal promoter elements in the tryptophan operon of Salmonella typhimurium. J Mol Biol. 1970 Apr 14;49(1):171–196. doi: 10.1016/0022-2836(70)90384-0. [DOI] [PubMed] [Google Scholar]
- Yang H. L., Kessler D. P. Genetic analysis of the leucine region in Escherichia coli B-r: gene-enzyme assignments. J Bacteriol. 1974 Jan;117(1):63–72. doi: 10.1128/jb.117.1.63-72.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Young B. G., Hartman P. E. Sites of P22 and P221 prophage integration in Salmonella typhimurium. Virology. 1966 Feb;28(2):265–270. doi: 10.1016/0042-6822(66)90150-4. [DOI] [PubMed] [Google Scholar]


