Abstract
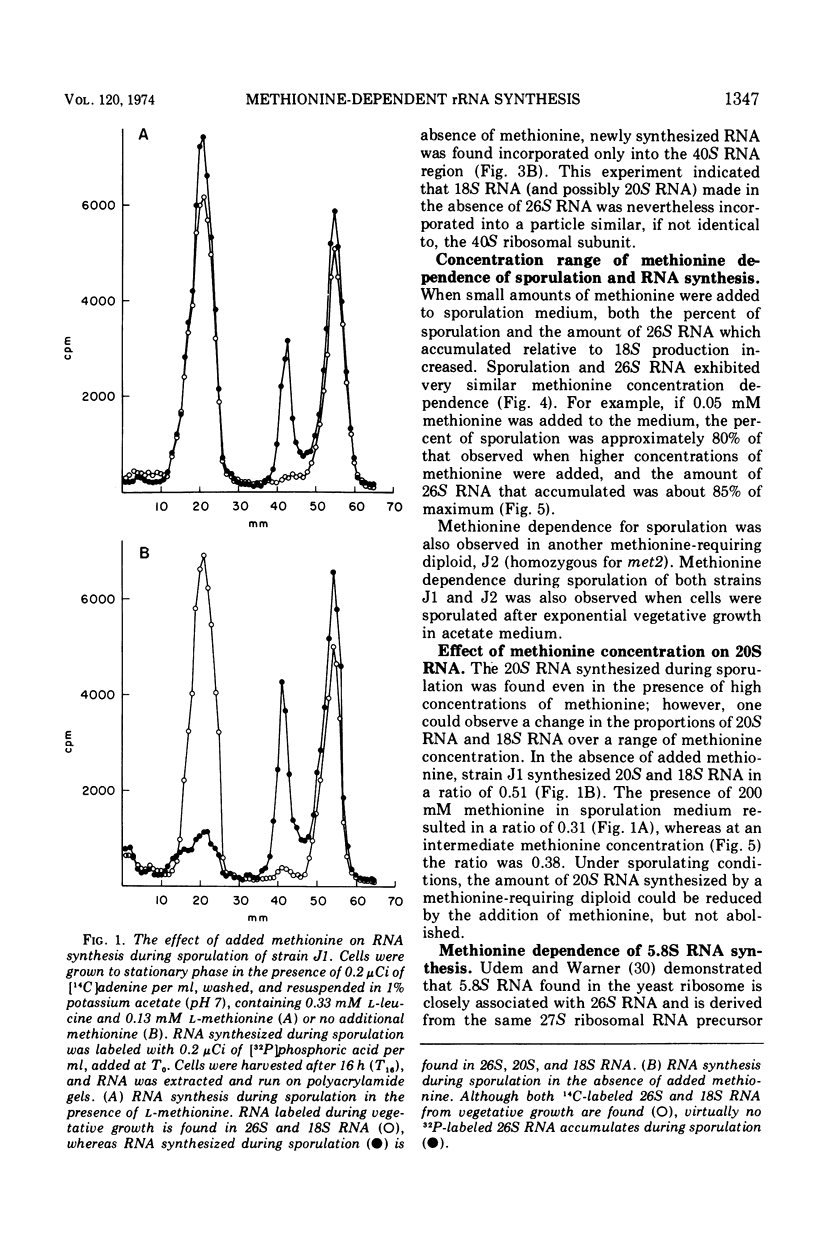
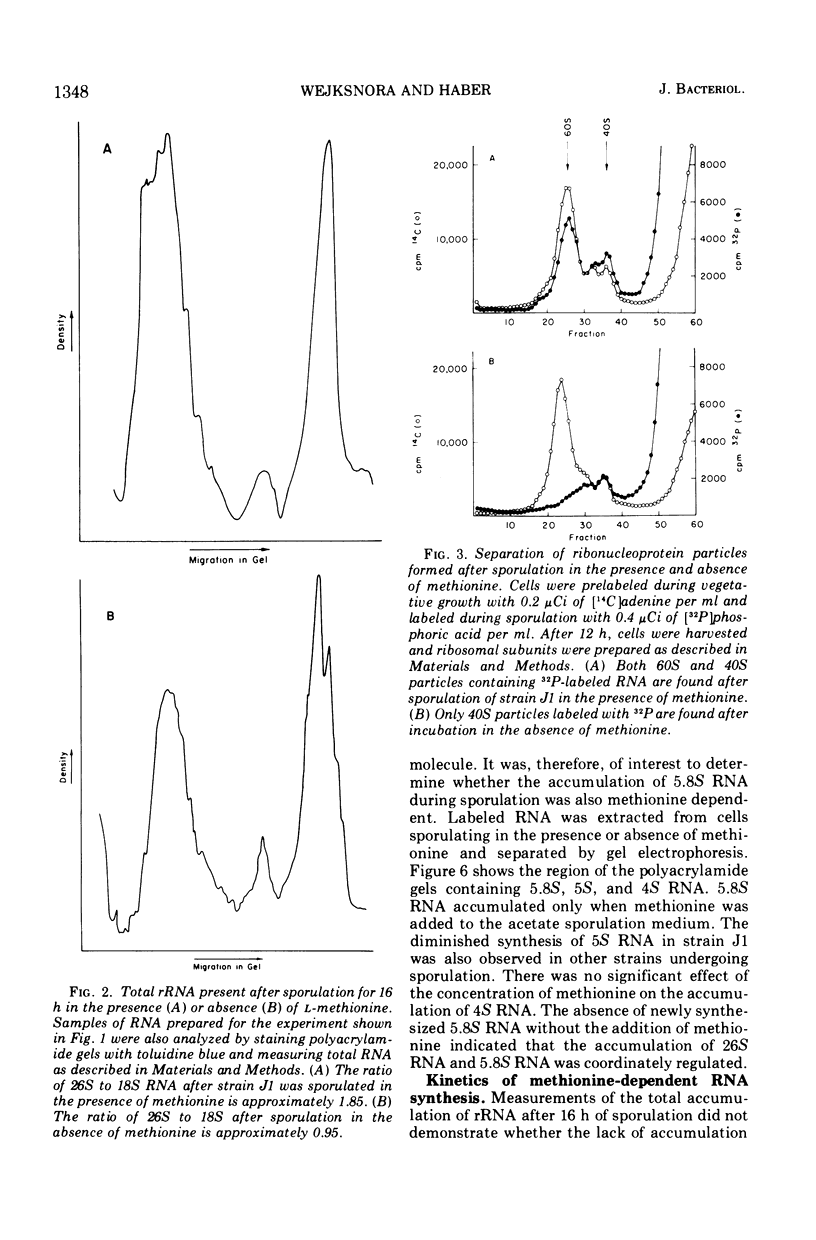
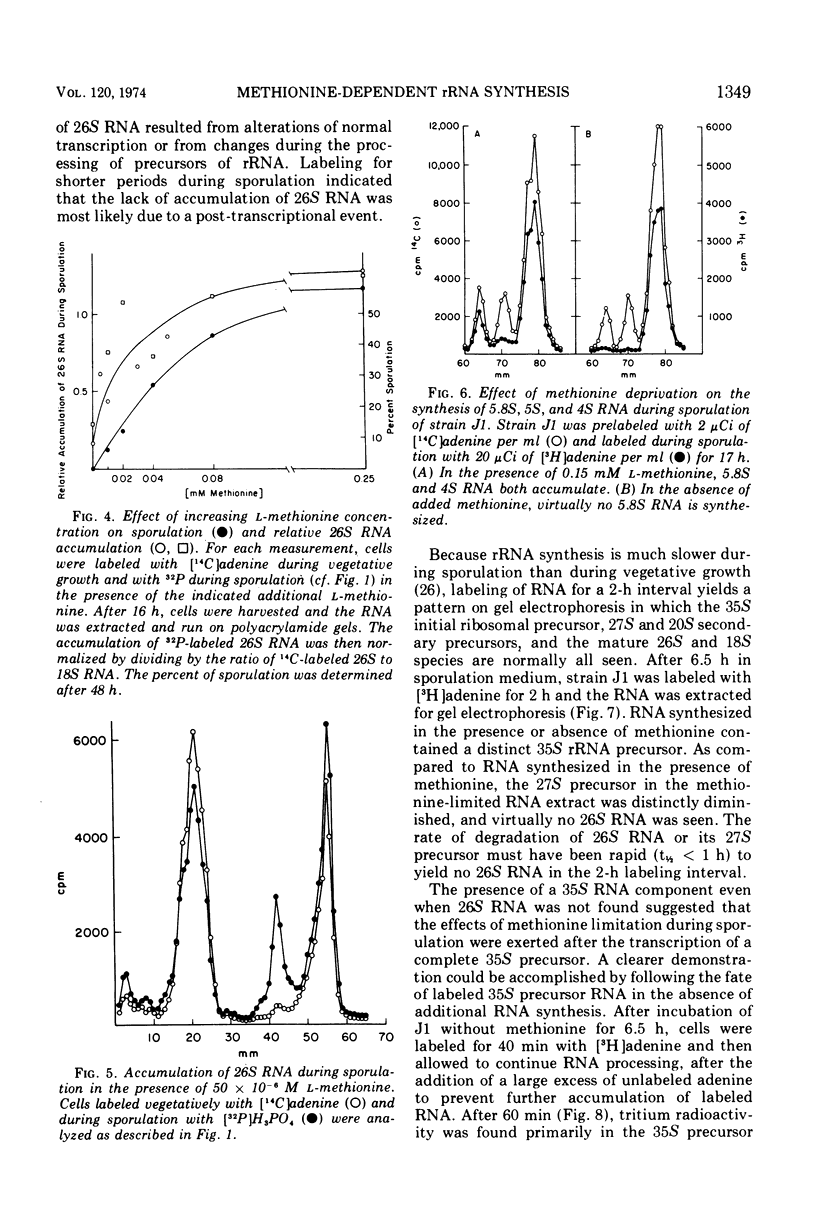
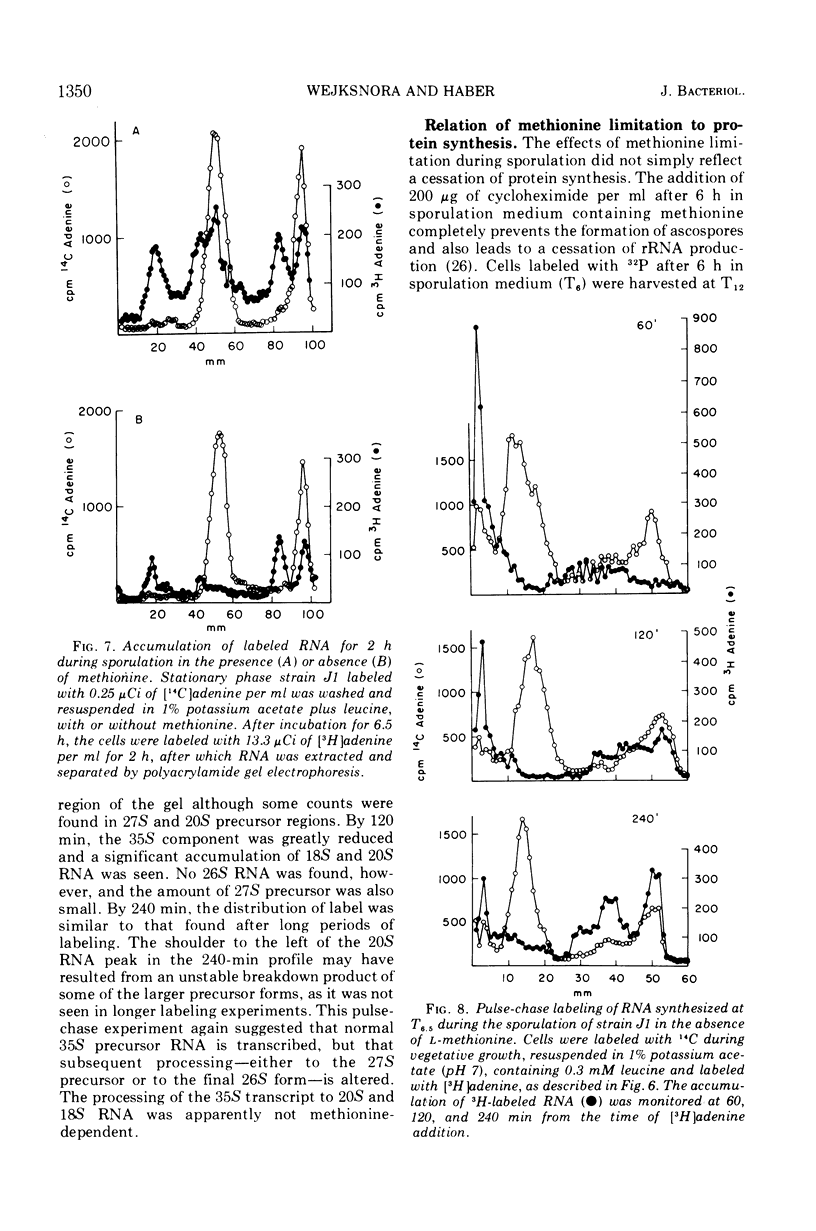
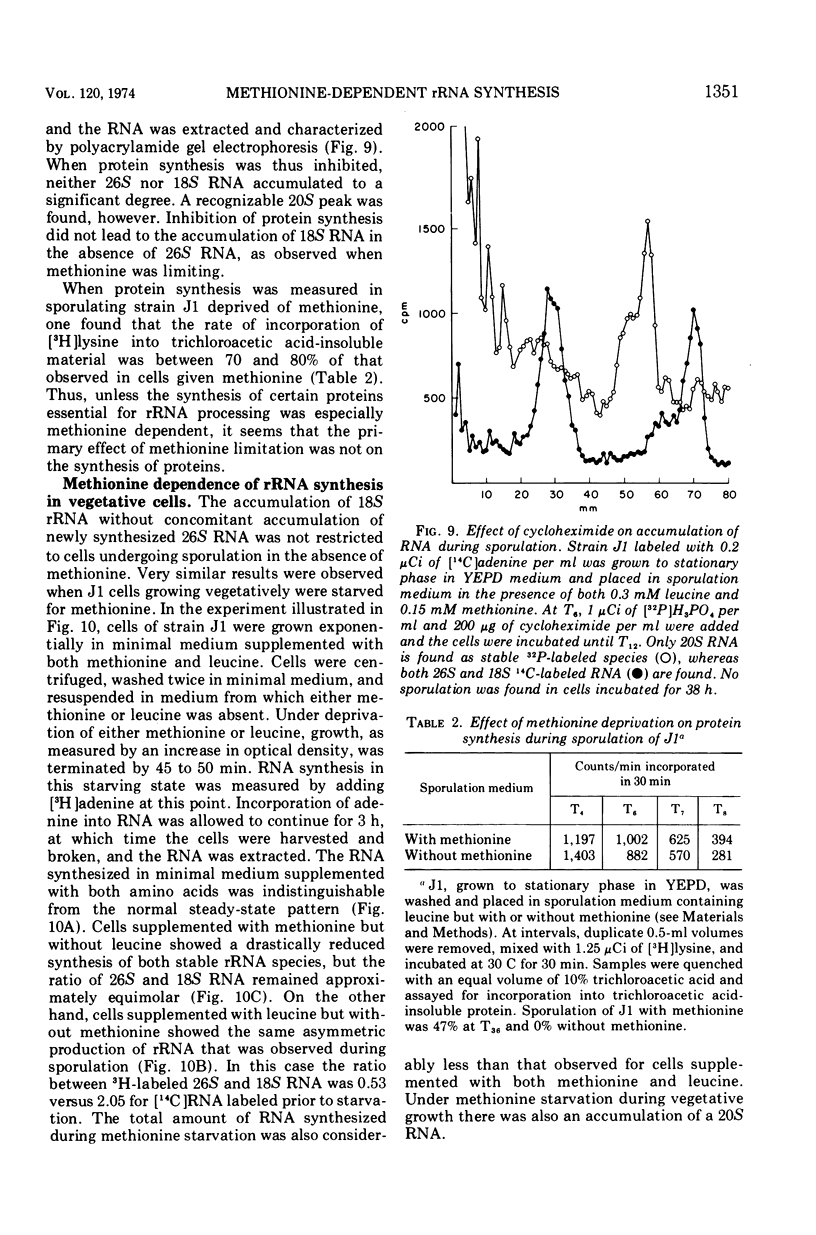
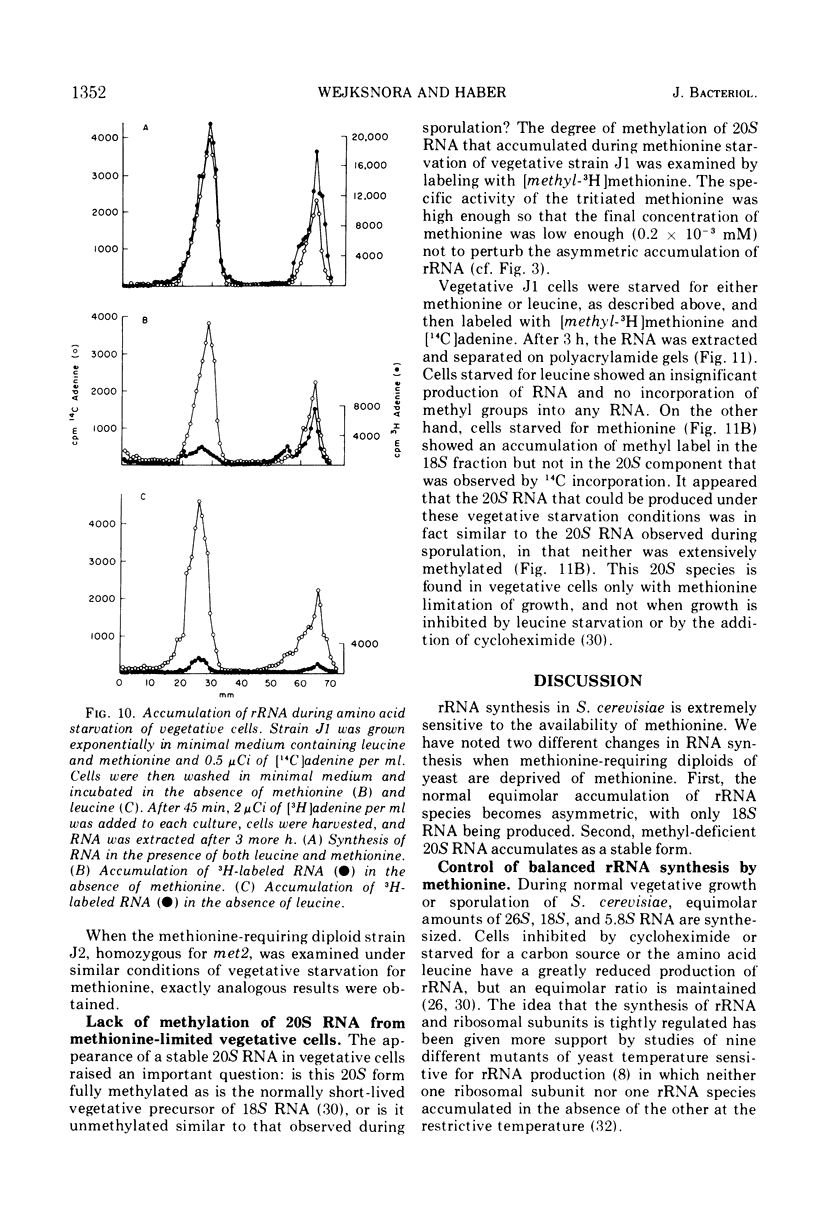
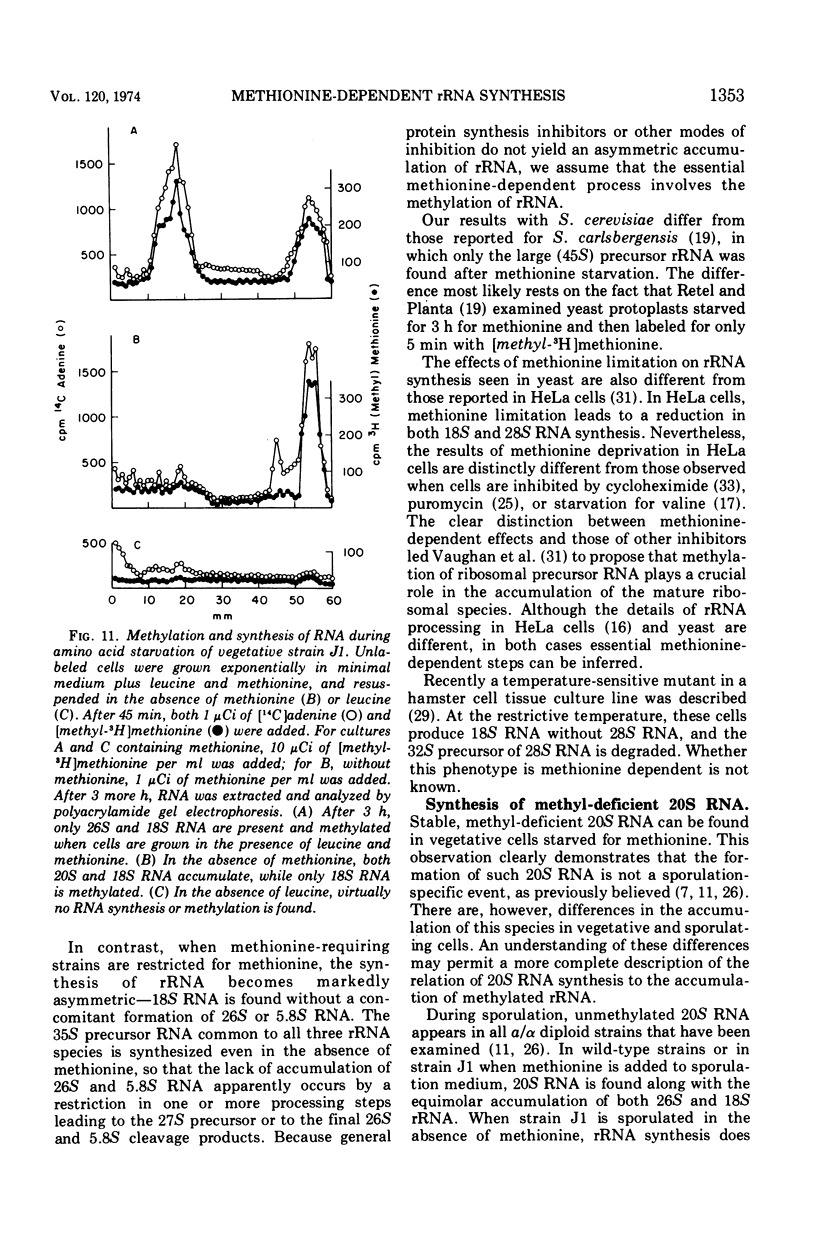
Methionine limitation during growth and sporulation of a methionine-requiring diploid of Saccharomyces cerevisiae causes two significant changes in the normal synthesis of ribonucleic acid (RNA). First, whereas 18S ribosomal RNA is produced, there is no significant accumulation of either 26S ribosomal RNA or 5.8S RNA. The effect of methionine on the accumulation of these RNA species occurs after the formation of a common 35S precursor molecule which is still observed in the absence of methionine. During sporulation, diploid strains of S. cerevisiae produce a stable, virtually unmethylated 20S RNA which has previously been shown to be largely homologous to methylated 18S ribosomal RNA. The appearance of this species is not affected by the presence or absence of methionine from sporulation medium. However, when exponentially growing vegetative cells are starved for methionine, unmethylated 20S RNA is found. The 20S RNA, which had previously been observed only in cells undergoing sporulation, accumulates at the same time as a methylated 18S RNA. These effects on ribosomal RNA synthesis are specific for methionine limitation, and are not observed if protein synthesis is inhibited by cycloheximide or if cells are starved for a carbon source or for another amino acid. The phenomena are not marker specific as analogous results have been obtained for both a methionine-requiring diploid homozygous for met13 and a diploid homozygous for met2. The results demonstrate that methylation of ribosomal RNA or other methionine-dependent events plays a critical role in the recognition and processing of ribosomal precursor RNA to the final mature species.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Cherest H., Surdin-Kerjan Y., Antoniewski J., Robichon-Szulmajster H. S-adenosyl methionine-mediated repression of methionine biosynthetic enzymes in Saccharomyces cerevisiae. J Bacteriol. 1973 Jun;114(3):928–933. doi: 10.1128/jb.114.3.928-933.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Esposito M. S., Esposito R. E., Arnaud M., Halvorson H. O. Acetate utilization and macromolecular synthesis during sporulation of yeast. J Bacteriol. 1969 Oct;100(1):180–186. doi: 10.1128/jb.100.1.180-186.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haber J. E., Halvorson H. O. Regulation of sporulation in yeast. Curr Top Dev Biol. 1972;7:61–83. doi: 10.1016/s0070-2153(08)60069-1. [DOI] [PubMed] [Google Scholar]
- Hartwell L. H., McLaughlin C. S., Warner J. R. Identification of ten genes that control ribosome formation in yeast. Mol Gen Genet. 1970;109(1):42–56. doi: 10.1007/BF00334045. [DOI] [PubMed] [Google Scholar]
- Henry S. A., Halvorson H. O. Lipid synthesis during sporulation of Saccharomyces cerevisiae. J Bacteriol. 1973 Jun;114(3):1158–1163. doi: 10.1128/jb.114.3.1158-1163.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hopper A. K., Magee P. T., Welch S. K., Friedman M., Hall B. D. Macromolecule synthesis and breakdown in relation to sporulation and meiosis in yeast. J Bacteriol. 1974 Aug;119(2):619–628. doi: 10.1128/jb.119.2.619-628.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kadowaki K., Halvorson H. O. Appearance of a new species of ribonucleic acid during sporulation in Saccharomyces cerevisiae. J Bacteriol. 1971 Mar;105(3):826–830. doi: 10.1128/jb.105.3.826-830.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kane S. M., Roth R. Carbohydrate metabolism during ascospore development in yeast. J Bacteriol. 1974 Apr;118(1):8–14. doi: 10.1128/jb.118.1.8-14.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klootwijk J., Planta R. J. Analysis of the methylation sites in yeast ribosomal RNA. Eur J Biochem. 1973 Nov 15;39(2):325–333. doi: 10.1111/j.1432-1033.1973.tb03130.x. [DOI] [PubMed] [Google Scholar]
- Küenzi M. T., Tingle M. A., Halvorson H. O. Sporulation of Saccharomyces cerevisiae in the absence of a functional mitochondrial genome. J Bacteriol. 1974 Jan;117(1):80–88. doi: 10.1128/jb.117.1.80-88.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loening U. E. The fractionation of high-molecular-weight ribonucleic acid by polyacrylamide-gel electrophoresis. Biochem J. 1967 Jan;102(1):251–257. doi: 10.1042/bj1020251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maden B. E., Salim M., Summers D. F. Maturation pathway for ribosomal RNA in the Hela cell nucleolus. Nat New Biol. 1972 May 3;237(70):5–9. doi: 10.1038/newbio237005a0. [DOI] [PubMed] [Google Scholar]
- Maden B. E., Vaughan M. H., Warner J. R., Darnell J. E. Effects of valine deprivation on ribosome formation in HeLa cells. J Mol Biol. 1969 Oct 28;45(2):265–275. doi: 10.1016/0022-2836(69)90104-1. [DOI] [PubMed] [Google Scholar]
- Mills D. Effect of pH on adenine and amino acid uptake during sporulation in Saccharomyces cerevisiae. J Bacteriol. 1972 Oct;112(1):519–526. doi: 10.1128/jb.112.1.519-526.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Retèl J., Planta R. J. Ribosomal precursor RNA in Saccharomyces carlsbergensis. Eur J Biochem. 1967 Dec;3(2):248–258. doi: 10.1111/j.1432-1033.1967.tb19524.x. [DOI] [PubMed] [Google Scholar]
- Retèl J., van den Bos R. C., Planta R. J. Characteristics of the methylation in vivo of ribosomal RNA in yeast. Biochim Biophys Acta. 1969 Dec 16;195(2):370–380. doi: 10.1016/0005-2787(69)90643-1. [DOI] [PubMed] [Google Scholar]
- Rodenberg S., Steinberg W., Piper J., Nickerson K., Vary J., Epstein R., Halvorson H. O. Relationship between protein and ribonucleic acid synthesis during outgrowth of spores of Bacillus cereus. J Bacteriol. 1968 Aug;96(2):492–500. doi: 10.1128/jb.96.2.492-500.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roman H., Sands S. M. Heterogeneity of Clones of Saccharomyces Derived from Haploid Ascospores. Proc Natl Acad Sci U S A. 1953 Mar;39(3):171–179. doi: 10.1073/pnas.39.3.171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roth R., Halvorson H. O. Sporulation of yeast harvested during logarithmic growth. J Bacteriol. 1969 May;98(2):831–832. doi: 10.1128/jb.98.2.831-832.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roth R., Lusnak K. DNA synthesis during yeast sporulation: genetic control of an early developmental event. Science. 1970 Apr 24;168(3930):493–494. doi: 10.1126/science.168.3930.493. [DOI] [PubMed] [Google Scholar]
- Sogin S. J., Haber J. E., Halvorson H. O. Relationship between sporulation-specific 20S ribonucleic acid and ribosomal ribonucleic acid processing in Saccharomyces cerevisiae. J Bacteriol. 1972 Nov;112(2):806–814. doi: 10.1128/jb.112.2.806-814.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Surdin-Kerjan Y., Cherest H., Robichon-Szulmajster H. Relationship between methionyl transfer ribonucleic acid cellular content and synthesis of methionine enzymes in Saccharomyces cerevisiae. J Bacteriol. 1973 Mar;113(3):1156–1160. doi: 10.1128/jb.113.3.1156-1160.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Toniolo D., Meiss H. K., Basilico C. A temperature-sensitive mutation affecting 28S ribosomal RNA production in mammalian cells. Proc Natl Acad Sci U S A. 1973 Apr;70(4):1273–1277. doi: 10.1073/pnas.70.4.1273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Udem S. A., Warner J. R. Ribosomal RNA synthesis in Saccharomyces cerevisiae. J Mol Biol. 1972 Mar 28;65(2):227–242. doi: 10.1016/0022-2836(72)90279-3. [DOI] [PubMed] [Google Scholar]
- Vaughan M. H., Jr, Soeiro R., Warner J. R., Darnell J. E., Jr The effects of methionine deprivation on ribosome synthesis in HeLa cells. Proc Natl Acad Sci U S A. 1967 Oct;58(4):1527–1534. doi: 10.1073/pnas.58.4.1527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Warner J. R., Udem S. A. Temperature sensitive mutations affecting ribosome synthesis in Saccharomyces cerevisiae. J Mol Biol. 1972 Mar 28;65(2):243–257. doi: 10.1016/0022-2836(72)90280-x. [DOI] [PubMed] [Google Scholar]
- Willems M., Penman M., Penman S. The regulation of RNA synthesis and processing in the nucleolus during inhibition of protein synthesis. J Cell Biol. 1969 Apr;41(1):177–187. doi: 10.1083/jcb.41.1.177. [DOI] [PMC free article] [PubMed] [Google Scholar]


