ABSTRACT
Even though meningiomas are most often benign tumors, they can be locally invasive and can develop in locations that prevent surgical treatment. The molecular and biologic factors underlying meningioma development are only now beginning to be understood. Genetic factors such as mutations in the neurofibromatosis-2 gene and in chromosomes 1, 9, and 10 play important roles in meningioma development and may be responsible for atypical tumors in some cases. Cellular factors such as telomerase activation and tyrosine kinase receptor mutations may also play an important role. Finally, autocrine and paracrine factors including epidermal growth factor receptor, platelet-derived growth factor-1, and fibroblast growth factor have been implicated in the development of some tumors. Although the relationship between the various factors implicated in tumor development is unknown, understanding these factors will be critical in the treatment of malignant or surgically inaccessible tumors.
Keywords: Aggressive, atypical, biology, meningioma, treatment
Meningiomas are typically viewed as benign tumors, with a favorable prognosis following surgical extirpation.1,2,3 In spite of this, however, many large studies with follow up have demonstrated that the 5-year mortality can be as high as 30 to 40%, with a median survival of 10 years.4,5,6 Furthermore, surgical resection of these tumors may also be associated with morbidity and mortality. The poorer prognosis for some subtypes of meningiomas is likely related to their biology (with aggressive histologic subtypes having a greater tendency to recur), location (i.e., cranial base), as well as proximity to vital neurovascular structures.7,8 Although morbidity from the latter two reasons will likely continue to decrease with advances in microsurgical technology and approaches, ultimately controlling the growth of aggressive and unresectable meningiomas will require an understanding of their growth, biology, and genetics. This article discusses our current understanding of the pathobiology for meningiomas and its application for treatment.
FACTORS RELATED TO MENINGIOMA INVASION AND GROWTH
Meningiomas are thought to be derived from the arachnoid cap cells and acquire blood supply from perforating vessels that arise from the external (most commonly) and internal carotid arteries, with a contribution from pial vessels.9 The degree of vascularity is highly variable between meningiomas, and as a consequence, their growth patterns and peritumoral edema can vary greatly, even in histologically benign tumors.
Vascular endothelial growth factor-A (VEGF-A), which has also been termed vascular permeability factor, is considered a key regulator of angiogenesis and edema formation. The VEGF-A mRNA is expressed by meningioma cells, and several studies demonstrated that VEGF-A levels in meningiomas are associated with the extent of peritumoral edema.10 Meningiomas with high VEGF-A expression also tend to recruit pial arterial supply and are associated with increased edema.11
Although some groups have suggested that VEGF-A transcription is associated with tumor vascularity, and that VEGF-A mRNA expression may correlate with meningioma vascularity,12 no study has confirmed the relationship between vessel density, VEGF level, and histologic grade.13 In spite of this, however, VEGF levels appear to be increased in atypical and anaplastic meningiomas and VEGF has been shown to promote blood vessel formation in vitro.13 These conflicting data likely indicate that VEGF-A is involved in vascular remodeling and angiogenesis in meningiomas but not necessarily in vascularity of histologic grade. VEGF-A might facilitate adaptation by modulating vascular permeability for the metabolic demands of aggressive tumors.
Several other growth factors, including VEGF-B, placenta growth factor, scatter factor/hepatocyte growth factor, and fibroblast growth factor-2, have also been analyzed in meningiomas, but no clear correlation between these factors and meningioma angiogenesis or histologic grade has been established. Expression of several other growth factors and their receptors also have been described,14 including platelet-derived growth factor (PDGF), fibroblast growth factor (FGF), and insulin-like growth factor (IGF), leukemia inhibitory factor (LIF), interleukin-6 (IL-6), oncostatin M (OSM), and epidermal growth factor (EGF).
PDGF-B is encoded by the oncogene c-sis in chromosome 22 and has been implicated in autocrine growth stimulation and malignant transformation of many tumors.15 Both PDGF-A and PDGF-B mRNA are detectable in meningiomas but only the PDGF-[β]-R receptor is expressed, which preferentially binds PDGF-BB. PDGF-BB (and rarely PDGF-AA) has been shown to stimulate cell growth and increase c-fos expression in meningioma cells in vitro.16,17
The role of other growth factors in meningioma growth remains poorly understood. FGF is a strong mitogen and angiogenic factor, and elevated expression of acidic and basic FGF in meningiomas has been reported, but the receptor expression was normal.18 IGF-II expression has been demonstrated in meningiomas but not normal brain or meninges and receptors have also been detected in tumors. The Ras group of signaling molecules transduce proliferative signals through tyrosine kinase receptors (TKRs) such as EGF receptor (EGFR) and PDGF-R. Ras inhibition using dominant negative constructs inhibits meningioma in vitro proliferation. Thus, inhibition of the Ras pathway may be important in preventing growth factor-stimulated meningioma proliferation and may be a target for therapy in the near future.19
There appear to be autocrine and paracrine effects that regulate meningioma growth as well. Meningiomas are more than twice as frequent in women than in men, indicating a possible role of estrogen or the estrogen receptor. Accelerated meningioma growth has also been observed during pregnancy and during the luteal phase of the menstrual cycle and tend to be slightly more common in patients with breast cancer.1
Immunohistochemical analysis has demonstrated that estrogen receptors are only expressed in ~10% of meningiomas at low levels.20,21 Progesterone and androgen receptors in comparison are present in approximately two thirds of meningiomas and are more frequently expressed in women.22,23 Although the effects of androgen antagonists on meningioma growth are unclear, progesterone receptor expression appears to be inversely associated with histological grade and the presence of progesterone receptors appears to be a favorable prognostic factor in meningioma patients.24 Furthermore, meningioma proliferation has been shown to be inhibited by a progesterone receptor antagonist, mifepristone (RU486), in vitro.21 However, clinical studies have been mixed, with mild clinical improvement in only 8 of 28 patients.25 Meningiomas also express nonsteroid hormone receptors, including growth hormone, somatostatin, and dopamine receptors. Several in vitro or experimental in vivo studies demonstrated antiproliferative effects of the growth hormone receptor antagonist pegvisomant, the somatostatin agonist octreotide, and the dopamine agonist bromocriptine on meningiomas.26
A subset of meningiomas have the ability to invade the brain or dura mater, leading to increased morbidity and a high rate of recurrence, despite gross total resection. The invasive potential of tumor cells is reflected by a balance between the expression of matrix metalloproteinases (MMPs) and tissue inhibitors of MMPs (TIMPs).27,28,29 This balance is probably not controlled by one single pathway, but rather by overlapping pathways. The group IV collagenases, MMP-2 and MMP-9, which degrade elements of the basement membrane, are expressed in 100% of meningiomas, with increased levels of expression in the higher grade tumors. One inhibitor of MMP-2 and MMP-9 activity, TIMP-1, was shown to be expressed in most (98%) meningiomas.
Another MMP inhibitor, tissue factor protein inhibitor-2 (TFPI-2) is an extracellular matrix associated kunitz-type serine protease inhibitor.30 Loss of TFPI-2 has been demonstrated in malignant gliomas and replacing TFPI-2 with TFPI-2 mRNA has been shown to decrease glioma invasiveness.31 Kondraganti et al32,33 found that restoration of TFPI-2 inhibits invasiveness and angiogenesis in a malignant meningioma cell line (IOMM-Lee) in vitro and in vivo.
Further work will be required to clarify the complex relationship between MMPs and TIMPs in the regulation of meningioma invasion.34 Table 1 provides a summary of factors associated with meningioma growth.
Table 1.
Types of Studies that Indicate Possible Factors and Their Postulated Mechanisms that Relate to Invasiveness and Tumor Growth of Meningiomas
| Postulated Mechanisms | Types of Supporting Studies | |
|---|---|---|
| MMP, matrix metalloproteinases; TIMP, tissue inhibitors of MMPs; PDGF, platelet-derived growth factor; EGFR, epidermal growth factor receptor; PDGFR, platelet-derived growth factor receptor. | ||
| Vascular endothelial growth factor-A (VEGF-A) | Angiogenesis and edema formation | Retrospective correlation of immunohistologic and |
| —magnetic resonance images p = 0.0001,34p = 0.0575 | ||
| —microvascular density count p = 0.000575 | ||
| In vitro correlation of chemotactic activity of VEGF on endothelial cells p = 0.00350 | ||
| Estrogen (E) | E receptors in 10–20% of meningiomas | Prospective immunohistological study of 57 meningiomas38 |
| Progesterone (PR) | PR receptors in ~67% of meningiomas, especially in women | Prospective immunohistological study of 57 meningiomas38 |
| Northern blot and in situ hybridization analysis in nine primary human meningiomas59 | ||
| Nonsteroidal hormones | Growth hormone, somatosatin, and dopamine receptors expressed in meningiomas | Review of immunohistological, in vivo, and in vitro studies76 |
| MMPs and TIMPs | Invasiveness correlated to increase in MMP:TIMP ratio | Immunohistologic studies comparing benign and anaplastic meningiomas7,26,77 |
| PDGF-β receptor | Autocrine growth stimulation and increase of c-fos expression | Northern blot in 20 meningiomas, measurement of exogenous PDGF-B on incubating cells of 10 meningiomas and measurement of c-fos expression11 |
| Comparison of astrocytomas, gliomas, and meningiomas57 | ||
| Fibroblast growth factor | Angiogenesis, mitogenic | Northern blot, in situ hybridization and immunohistochemistry studies of 22 meningiomas compared with metastatic cancers and gliomas94 |
| Ras | Transduce proliferative signals via EGFR and PDGFR | Inoculation of nine meningioma cell cultures with recombinant adenovirus Ad-rasN1790 |
GENETICS OF MENINGIOMAS
Chromosonal Abnormalities and Tumor Suppressor Genes
Although meningiomas were among the earliest tumors to have been described pathologically and studied,35 the pathophysiology of meningiomas is still poorly understood. The initial clue into genetic alterations associated with meningiomas came from examining patients with neurofibromatosis-type 2 (NF-2). In the early 1970s, the candidate NF-2 gene was cloned and named schwannomin/MERLIN (moesin-ezrin-radixin-like protein).36,37,38,39,40,41,42 Shortly after its initial description, the gene was localized to chromosome 22 using linkage analysis.43,44,45 The NF-2 gene is a part of the “Protein 4.1” family of tumor suppressors. Loss of heterozygosity (LOH) of chromosome 22 is detected in 95% of fibroblastic meningiomas but only 33% of meningothelial meningiomas. Analysis of the NF-2 protein showed differential expression related to meningioma histology, with reduced expression in 28.5% of meningothelial meningiomas but 86% of other subtypes.46
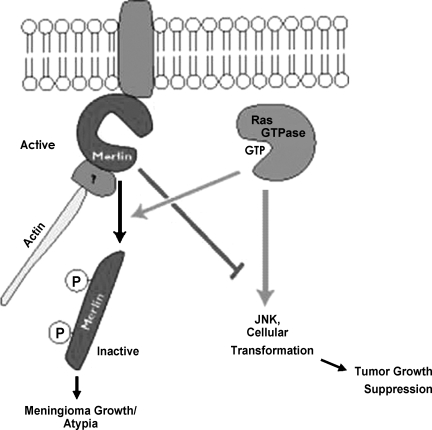
Modern molecular techniques have demonstrated that the mutation was not only found in meningiomas in patients with NF-2, but also in the majority of patients with sporadic meningiomas.47,48,49,50,51,52 Most mutations occur in tumors that have already lost one chromosome 22 allele, supporting the “two-hit” hypothesis for tumorigenesis (Fig. 1).53,54
Figure 1.
The interaction between the neurofibromatosis factor-2 tumor suppressor, merlin, and the meningioma growth demonstrates the effect of a tumor suppressor gene. Cytoskeleton-bound merlin attenuates Ras-mediated JNK activation and results in cellular transformation. Ras-induced phosphorylation of merlin inactivates the tumor suppressor and promotes its dissociation from the cytoskeleton and results in tumor growth. NF-2, neurofibromatosis-2; GTP, guanine triphosphate; JNK, jun N-terminal kinase.
A variety of explanations exist for meningiomas that are not associated with a NF-2 mutation. In some of these cases it has been postulated that conventional techniques for detecting gene mutations miss the NF-2 mutation, or that NF-2 is inactivated by mechanisms of methylation. Alternatively, it is equally plausible that there is a large subset of meningiomas that do not harbor the NF-2 mutation.55 In these cases, other tumor suppressor genes associated with meningioma tumorigenesis have been found at various locations, including the long arm of chromosome 22, as well as other chromosomes. Altered expression of another Protein 4.1 family member on chromosome 18, DAL-1, has been identified in more than three quarters of meningiomas by immunohistochemistry and is often associated with NF-2 loss.56 Further investigations have shown that lack of DAL-1 protein was slightly, but not significantly, more frequent in anaplastic meningiomas (87%) when compared with benign and atypical meningiomas (70 to 76%), suggesting that its deletion could represent an early event in meningioma tumorigenesis.56 These immunohistochemical findings were largely reflected at the mRNA expression level as well. The mechanism of DAL-1 inactivation is still unknown. Mutations have not been detected, but screening has not included the full genome.57 Given the frequent LOH at the DAL-1 gene location, homozygous deletions are unlikely, so that epigenetic alterations remain a more likely possibility.
Different gene families, including 1p, 9q, 10q, and 17q, have also been found more frequently in meningiomas associated with radiation exposure when compared with sporadic tumors,58 and are summarized below.
CHROMOSOME 1
Deletions on 1p are the second most frequent chromosomal abnormality in meningiomas. The frequency of 1p deletions increases with tumor grade and occurs in 13 to 26% of grade 1 tumors, in 40 to 76% of grade II tumors, and in 70 to 100% of grade 3 tumors. Moreover, 1p deletions were found to be associated with tumor progression in several individual cases of recurrent meningiomas.59,60 These findings suggest that loss of genomic information from 1p is relevant to meningioma progression rather than tumor formation.
Several genes on 1p were screened for alterations in meningiomas, including CDKN2C (p18INK4c), p73, RAD45L, and ALPL. However, pathologic studies on meningioma tissues have not demonstrated statistically significant correlation between gene deletion and meningioma subtype.61,62,63
Another candidate meningioma suppressor gene is TP73, which maps to 1p36.32. However, only 1 mutation was found in more than 50 meningiomas analyzed, which argues against a significant role of TP73 inactivation in meningioma progression.64,65 Expression of TP73 was found to increase with tumor grade, suggesting that TP73 might have a dominant oncogenic function rather than a classic tumor suppressor function.66
Thus the analyses of individual genes on 1p do not support a significant meningioma suppressor function of any of the genes investigated. Deletion mapping studies defined at least two different commonly deleted regions on 1p, suggesting that more than one tumor suppressor gene on 1p might be involved in meningioma progression. One of these regions was mapped to 1p32.67 However, sequence information that became available through the human genome project indicates that mapping data that were previously interpreted mainly on the basis of recombination events require revision in several instances.14,67
A second common region of deletion was mapped to 1pter-p34 distally to the D1S496 locus.68 According to current databases, this microsatellite still maps to 1p34.3 and consequently there is less confusion for this region than for the more proximal region. A terminally located region of deletion at 1p36 has also been described, with three other commonly deleted regions, including a frequently affected region at 1p34-p32.59,60
Taken together, it appears that at least two common regions of deletion are present on chromosome arm 1p, namely one at 1p34-p32 and another at 1pter-p34. It remains to be shown whether reanalysis of previous studies based on genomic sequence information can clarify some of the confusing mapping data and can narrow down regions containing putative tumor suppressor genes. Naturally, the current confusion is not unique to meningiomas, since deletions on 1p are also common in oligodendrogliomas, neuroblastomas, and many epithelial cancers.20
CHROMOSOME 9
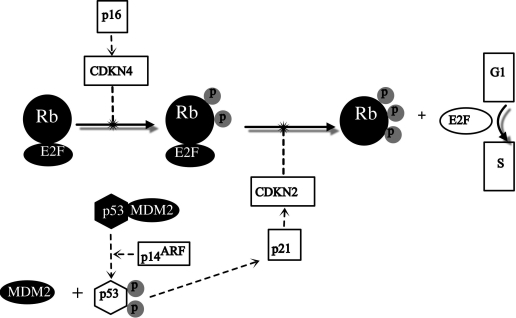
Losses of genetic material on chromosome 9 have been frequently found in malignant meningiomas, but only rarely in benign or atypical ones. The short arm of chromosome 9 has attracted particular attention, as it contains the tumor suppressor genes CDKN2A (p16INK4a/MTS1), p14ARF, and CDKN2B (p15INK4b/MTS2) at 9p21, which are inactivated at a high frequency in a variety of human tumors. The p16 and p15 proteins regulate cell cycle progression at the G1/S-phase checkpoint by inhibiting cyclin-cdk complexes, which are involved in the transcriptional control and phosphorylation of pRB. The p14ARF protein blocks Mdm2-mediated degradation of p53 (Fig. 2).
Figure 2.
The figure shows the role of CDKN2 and p14ARF at 9p21 and p16 in suppression of proteins that mediate progression of G1 to S phase. Rb is phosphorylated to activate E2F which mediates transcription of genes necessary for G1 to S phase progression. Malignant meningiomas have been found to have inactivated CDKN2, p14ARF, and p16, which rescinds tumor suppression. Solid lines represent progression of malignant meningioma whereas dotted lines are G1/S checkpoint pathways.
Losses on 9p, as determined by combined comparative genomic hybridization (CGH) and microsatellite analysis, were found in 38% of grade 3 meningiomas, compared with only 5% of grade 1 tumors.61 Moreover, fluorescent in situ hybridization (FISH) analysis has demonstrated the frequency of deletion at 9p21 or monosomy 9 to be two- to threefold higher in aggressive tumors.69,70 Furthermore, the majority of malignant meningiomas have been shown to display alterations of CDKN2A, p14ARF, and CDKN2B, whereas these were infrequent in benign meningiomas.61,71,72,73,74 Similarly, in an expression analysis by Simon et al,74 only ~26% of grade 1 and 2, but 57% of grade 3 tumors lacked both p16 and p15 protein expression. In addition, 36% of nonmalignant but 71% of the anaplastic meningiomas lacked p14 protein. These studies show that both the pRB and p53 pathways are disrupted in the majority of high grade meningiomas, and that inactivation of cell cycle regulation at the G1/S-phase checkpoint may be critical to the malignant progression of meningiomas.
CHROMOSOME 10
Deletions on chromosome 10 have received considerable attention in meningiomas. Rempel et al75 initially discovered an association between allelic losses on chromosome 10 with meningioma progression. Most subsequent LOH or CGH analyses detected deletions on the long arm of chromosome 10.76 Correlative analyses revealed an unfavorable prognostic significance for LOH at D10S179 (1p14) or D10S169 (1q26.3), which predicted higher tumor grade, and of D10S209 (10q26.12) and D10S169 (10q26.3), which may predict shorter survival and/or shorter time to recurrence, respectively.77
No specific tumor suppressor gene on chromosome 10 has yet been shown to be inactivated in a major fraction of meningiomas. The PTEN gene at 10q23.3 was analyzed in a large number of samples; however, mutations were only detected in two grade 3 tumors, with no evidence of homozygous deletions.34,78
Mapping studies defined several different commonly deleted regions on chromosome 10.79 However, these studies indicate that the pattern of allelic losses on chromosome 10 is so complex that no single consistent minimal region of deletion can be identified.
CHROMOSOME 14
Cytogenetically, loss of chromosome 14 represents the third most frequently detected abnormality in meningiomas after aberrations of chromosomes 22 and 1.80,81 Indeed, abnormalities in chromosome 14 are seen in 100% of grade 3 meningiomas, compared with 31% of grade 1 tumors.80,81 The strongly increased frequency of 14q deletions in tumors of higher grade suggests an involvement in meningioma progression. A recent study demonstrated that deletions on this chromosome arm were an independent adverse prognostic parameter, which when combined with histological grade and patient age could identify patients at increased risk of relapse.82
To date, only one specific meningioma suppressor gene has been identified on chromosome 14. Lusis et al83 found expression of NDGR2, located at 14q11.2, to be downregulated in 40% of anaplastic meningiomas and atypical meningiomas with aggressive behavior. This downregulation was found to be the result of promoter hypermethylation.83
CHROMOSOME 17
The tumor suppressor gene TP53, which is located on the short arm of chromosome 17, is one of the most frequently mutated genes in human cancer in general and in astrocytomas in particular. However, TP53 mutations are rare in meningiomas. Several relatively large studies reported either no or only single meningiomas with sporadic TP53 mutations.84,85 Mutation of TP53 frequently results in increased stability of the p53 protein, which, in contrast to wild-type p53, then becomes detectable by immunohistochemistry. Other stabilization mechanisms may also lead to p53 accumulation and thus detectability. Immunohistochemical analyses of p53 in meningiomas have yielded contradictory results, and there seems to be a substantial degree of overlap between different malignancy grades. Most studies have demonstrated an association between p53 accumulation and malignancy grade.86,87 The biological significance of this p53 accumulation in meningiomas is not clear.
More recently, the long arm of chromosome 17 has also attracted interest since CGH analysis demonstrated high-level amplification on 17q in 42% of anaplastic meningiomas but in almost none of the nonmalignant meningiomas.76 However, in contrast to another recent study by Cai et al,62 which identified PS6K amplification in 3 of 22 anaplastic meningiomas, only low-level copy number increases were detected in 13 of 44 tumors despite high-level amplification of adjacent loci in two high grade (grade 3) tumors. This discrepancy could have technical reasons, because Cai et al used FISH analysis, which recognizes also only focal areas with high-level PS6K amplification, whereas focal high-level amplifications may have appeared as low-level amplification in the real-time PCR analysis performed by Büschges et al.88
Oncogenes
The role of oncogene activation in meningioma pathogenesis is poorly defined. As with other tumors, there appears to be a step-wise process of tumor progression, with gradual loss of tumor suppressor genes and activation of oncogenes,55 with atypical and malignant tumors being more likely to have genetic aberrations when compared with benign tumors. Progression from benign meningioma with losses on chromosome 22q to atypical or malignant meningioma is associated with the accumulation of abnormalities on several additional chromosomes, particularly losses on 1p, 2p, 6q, 10, 14q, and 18q, and gains on 20q, 12q, 15q, 1q, 9q, and 17q.34,75,76,89 Losses on 1p and 14q are particularly associated with the progression from benign to atypical or malignant meningioma.90
Like the NF-2 gene, the c-sis oncogene maps to chromosome 22 and encodes the B-chain of PDGF-B. High levels of expression of this gene have been detected in some meningiomas,91,92 but the gene is not amplified and the role of c-sis is unclear.15 C-myc, Ha-ras, K-ras, and c-fos are also overexpressed or amplified in some tumors and may contribute to growth factor autocrine loop signaling. C-erb B, which encodes part of the EGF receptor (EGFR), is also detectable in meningiomas but it is not amplified and EGFR may be largely localized to vascular endothelium rather than tumor cells.15,93
Progression from benign to anaplastic histology in meningiomas has also recently been associated with telomerase activity.94 Telomerase is a ribonucleoprotein enzyme responsible for replicating telomeric DNA. It has been shown to be reactivated in several cancers, including malignant glioma. Telomerase activity, as well as expression of hTERT mRNA (which encodes the catalytic subunit of human telomerase reverse transcriptase) has been found in the overwhelming majority of anaplastic meningiomas, as well as most atypical meningiomas, while the activity is significantly less in benign meningiomas.95
FUTURE DIRECTIONS IN MENINGIOMA THERAPY
Although surgical resection will remain the mainstay in meningioma treatment, tumor location and biological characteristics can prevent a complete surgical cure. Although adjuvant treatment modalities, such as radiosurgery, systemic medications, and whole beam radiation have individual benefits, all of these modalities have known side effects, and are not options for some patients. Newer methods of meningioma treatment will rely on understanding and utilizing the molecular biology of these tumors to arrest growth. Some promising new therapies are outlined below.
Angiogenesis Inhibitors
Vascular inhibition using interferon-α, a leukocyte-produced cytokine that is related to transforming growth factor β and tumor necrosis factor, has been investigated as a mechanism of halting meningioma growth. Interferons work by their antiangiogenic effect and by directly blocking tumor cell proliferation.96 Interferon has been reported to have an effect on meningiomas in vivo and in vitro.97,98,99,100 Kaba et al97 reported on 6 patients with recurrent or malignant meningiomas treated with interferon α. Five of the 6 patients had stabilization of disease progression, with the duration of tumor stabilization ranging from 6 to 14 months. Muhr et al101 examined meningioma metabolism in 12 patients treated with interferons. This group followed patients with serial [C]-L-methionine uptake positron emission tomography studies, and demonstrated stabilization of disease in 9 patients. In both reports, interferon treatment toxicities were tolerable, with patients complaining mostly of flu-like symptoms and leukopenia.
Endothelins (ET) are peptides composed of 21 amino acids with three identified isoforms (ET-1, ET-2, and ET-3). They exert their effects via two receptor subtypes: ET-A and ET-B. Endothelins are angiogenic, potent vasoconstrictors (via ET-A receptors) and vasodilators (via ET-B receptors), and they also incite mitogenesis and c-Fos expression of neuronal cell types in vivo.102,103 Thus, ET has been hypothesized to promote angiogenesis and to act as an autocrine/paracrine growth factor in cerebral tumors. Harland et al104,105 found that meningiomas expressed a higher level of ET-A receptors compared with normal cortex, and they concluded that the high affinity ET-A receptor antagonist PD156707 may be of therapeutic value in treating these lesions.103 Verotoxin has recently been studied as a new antineoplastic agent that targets the globotriaosylceramide (Gb3) glycolipid on tumor cells and tumor neovasculature. Verotoxins, or Shiga-like toxins, are produced by Escherichia coli and are associated with the pathogenesis of hemolytic uremia syndrome, hemorrhagic colitis, and microangiopathies. Verotoxin is a type II ribosome-inactivating protein produced by pathogenic strains of E. coli and targets only cells that express glycolipid Gb3 (CD77). Gb3 has been noted to be unregulated in many human cancers. The toxin consists of an A-subunit (enzyme) and B-subunit (antigen). The B-subunit recognizes specific glycolipids in particular cells, which are known to be elevated in several human cancers. Salhia and associates106 demonstrated that the verotoxin receptor was present in 9 of 11 (82%) malignant meningiomas and that intratumoral injection of verotoxin in a mouse xenograft model resulted in increased survival of seeded animals. Microscopically, the verotoxin-treated animals showed decreased microvascular density, increased apoptosis, and a decrease in meningioma cell proliferation.
Growth Hormone Antagonists
Interest in the roles that growth hormone and insulin-like growth factor I (IGF-I) play in meningiomas' tumorigenesis stems first from observations that patients with acromegaly have a high incidence of meningiomas (1.5% in one large series), and from studies showing involvement of IGF-I in meningioma growth in cultures.107 Growth hormone is produced and secreted from the anterior pituitary and stimulates the synthesis of IGF-I in the liver, the combined effects of which result in normal growth. In vivo and in vitro studies have shown that the growth hormone receptor is ubiquitous in meningiomas and that blockade results in decreased tumor growth.108 Pegvisomant is a genetically engineered protein designed to be structurally similar to the natural human growth hormone. It is capable of binding to the growth hormone receptor, acting as a competitive antagonist. Friend et al109 examined 14 human meningioma specimens that were grown in primary culture. They found the ubiquitous expression of growth hormone receptor mRNA in all 14 specimens, regardless of tumor grade (benign, anaplastic, or malignant). Blockade of the growth hormone receptor with pegvisomant reduced serum induced DNA synthesis as measured by thymidine incorporation by 8 to 33% (mean, 20%). IGF-I increased thymidine incorporation in primary meningioma cultures in a dose-dependent manner: 1 ng/mL, 5 ng/mL, and 10 ng/mL resulted in 21%, 43%, and 176%, respectively, above baseline. Based on the above study, McCutcheon and colleagues108 examined five human meningioma tumors and implanted them into the flanks of nude mice. After 8 weeks, the mean tumor volume of the pegvisomant group was 198.3 ± 18.9 mm3 versus 350.1 ± 23.5 mm3 for the control group (P < .001). The serum IGF-I concentration in the control group was 319 ± 12.9 μg/L compared with 257 ± 9.7 μg/L in the pegvisomant group (P < .02). The effects noted in this in vivo tumor model were most likely due to both the decrease in circulating IGF-I and the direct blockade of meningioma tumor growth hormone receptors. Furthermore, clinical trials have studied the treatment of acromegaly with pegvisomant and have shown that this drug is well tolerated by patients.110
Signal Transduction Pathway Inhibition
The growth of meningiomas in culture and the potent growth stimulation of meningioma cells by EGF and PDGF are inhibited by calcium channel antagonists.111,112 These studies arose out of the observation that calcium is an integral component of the intracellular signaling pathways. It was hypothesized that calcium channel antagonists might interrupt this signaling with subsequent growth inhibition. These studies and others concluded that the activity of the calcium channel antagonist agents had nothing to do with calcium signaling.113 Nevertheless, these studies were expanded into animal studies, allowing for the growth of meningiomas in a xenograft model. Animals with subcutaneous flank meningiomas were treated with verapamil and diltiazem. Serum drug levels of these medications verified that the drugs were reaching concentrations that would be found in patients being treated for hypertension. Growth inhibition was observed, but the results were modest. No long-term “cures” were found. There is evidence that calcium channel antagonists can potentiate the effects of chemotherapeutic drugs. Ragel and Jensen26 found that the addition of verapamil or diltiazem to hydroxyurea or RU486 augmented the growth inhibition and induced apoptosis in one malignant (IOMM-Lee) and six benign human mengioma lines in vitro. The effect of the calcium channel antagonists worked via inhibition of cell cycle progression, arresting the cells in the G1-phase (as determined by flow cytometry analysis). In vivo (and on the same cell lines), the same combination of drugs slowed proliferation and decreased microvascular density. Human glioma tumor growth is inhibited by verapamil alone and more dramatically in combination with the chemotherapeutic agent 1,3-bis(2-chloroethyl)-1-nitrosourea (BCNU) both in vitro and in vivo.114 In fact, the cytotoxicity of many different standard anticancer agents is augmented by calcium channel antagonists in several tumor cell types.115,116,117
Gene Therapy
Gene therapy is an emerging technology that offers a therapeutic option for incurable brain tumors.118 Chauvet et al119 successfully infected a canine meningioma with a recombinant adenovirus vector via selective intra-arterial injection. A specific target for gene therapies in patients afflicted with meningiomas associated with NF-2 is transfection with merlin, the wild-type NF-2 gene. Ikeda et al120 showed the feasibility of gene transfer by infecting the wild-type NF-2 transgene into human meningioma tumors excised from patients with and without NF-2, using a herpes simplex virus. Western blot analysis confirmed that vector-mediated gene transfer mediated the expression of the NF2-encoded polypeptide merlin and that overexpression of merlin significantly inhibited the growth of both NF-2–negative and NF-2–positive human meningioma cells when compared with controls. Dirven et al79 proved that the adenovirus viral vector could be altered to target specific tumor receptors on meningiomas, thus enhancing gene transfer.
CONCLUSIONS
Advances in molecular biology provide unique insights into the pathobiology of meningiomas. Understanding the interplay between these differing mechanisms will allow treatment of these tumors in the future.
REFERENCES
- Campan L, Zadeh J. Age and other factors in the prognosis and operative indications for brain surgery [in French] Ann Anesthesiol Fr. 1977;18(5–6):475–480. [PubMed] [Google Scholar]
- Bonnal J, Sedan R, Pellet W, et al. Operative prognosis of benign intracranial tumors (study of 282 cases) [in French] Neurochirurgie. 1966;12:361–371. [PubMed] [Google Scholar]
- Ahn E S, Chin L S, Gyure K A, et al. Long-term control after resection and gamma knife surgery of an intracranial clear cell meningioma: case report. J Neurosurg. 2005;102(suppl):303–306. doi: 10.3171/ped.2005.102.3.0303. [DOI] [PubMed] [Google Scholar]
- Sankila R, Kallio M, Jaaskelainen J, Hakulinen T. Long-term survival of 1986 patients with intracranial meningioma diagnosed from 1953 to 1984 in Finland: comparison of the observed and expected survival rates in a population-based series. Cancer. 1992;70:1568–1576. doi: 10.1002/1097-0142(19920915)70:6<1568::aid-cncr2820700621>3.0.co;2-y. [DOI] [PubMed] [Google Scholar]
- Kallio M, Sankila R, Jaaskelainen J, Karjalainen S, Hakulinen T. A population-based study on the incidence and survival rates of 3857 glioma patients diagnosed from 1953 to 1984. Cancer. 1991;68:1394–1400. doi: 10.1002/1097-0142(19910915)68:6<1394::aid-cncr2820680636>3.0.co;2-8. [DOI] [PubMed] [Google Scholar]
- Mirimanoff R O, Dosoretz D E, Linggood R M, Ojemann R G, Martuza R L. Meningioma: analysis of recurrence and progression following neurosurgical resection. J Neurosurg. 1985;62:18–24. doi: 10.3171/jns.1985.62.1.0018. [DOI] [PubMed] [Google Scholar]
- Davis F G, McCarthy B J, Freels S, Kupelian V, Bondy M L. The conditional probability of survival of patients with primary malignant brain tumors: surveillance, epidemiology, and end results (SEER) data. Cancer. 1999;85:485–491. [PubMed] [Google Scholar]
- McCarthy B J, Davis F G, Freels S, et al. Factors associated with survival in patients with meningioma. J Neurosurg. 1998;88:831–839. doi: 10.3171/jns.1998.88.5.0831. [DOI] [PubMed] [Google Scholar]
- Bitzer M, Wockel L, Luft A R, et al. The importance of pial blood supply to the development of peritumoral brain edema in meningiomas. J Neurosurg. 1997;87:368–373. doi: 10.3171/jns.1997.87.3.0368. [DOI] [PubMed] [Google Scholar]
- Goldman C K, Bharara S, Palmer C A, et al. Brain edema in meningiomas is associated with increased vascular endothelial growth factor expression. Neurosurgery. 1997;40:1269–1277. doi: 10.1097/00006123-199706000-00029. [DOI] [PubMed] [Google Scholar]
- Provias J, Claffey K, delAguila L, Lau N, Geldkamp M, Guha A. Meningiomas: role of vascular endothelial growth factor/vascular permeability factor in angiogenesis and peritumoral edema. Neurosurgery. 1997;40:1016–1026. doi: 10.1097/00006123-199705000-00027. [DOI] [PubMed] [Google Scholar]
- Samoto K, Ikezaki K, Ono M, et al. Expression of vascular endothelial growth factor and its possible relation with neovascularization in human brain tumors. Cancer Res. 1995;55:1189–1193. [PubMed] [Google Scholar]
- Lamszus K, Lengler U, Schmidt N O, et al. Vascular endothelial growth factor, hepatocyte growth factor/scatter factor, basic fibroblast growth factor, and placenta growth factor in human meningiomas and their relation to angiogenesis and malignancy. Neurosurgery. 2000;46:938–947. discussion 947–948. doi: 10.1097/00006123-200004000-00033. [DOI] [PubMed] [Google Scholar]
- Leuraud P, Marie Y, Robin E, et al. Frequent loss of 1p32 region but no mutation of the p18 tumor suppressor gene in meningiomas. J Neurooncol. 2000;50:207–213. doi: 10.1023/a:1006400723490. [DOI] [PubMed] [Google Scholar]
- Maxwell M, Galanopoulos T, Hedley-Whyte E T, Black P M, Antoniades H N. Human meningiomas co-express platelet-derived growth factor (PDGF) and PDGF-receptor genes and their protein products. Int J Cancer. 1990;46:16–21. doi: 10.1002/ijc.2910460106. [DOI] [PubMed] [Google Scholar]
- Black P M, Carroll R, Glowacka D, Riley K, Dashner K. Platelet-derived growth factor expression and stimulation in human meningiomas. J Neurosurg. 1994;81:388–393. doi: 10.3171/jns.1994.81.3.0388. [DOI] [PubMed] [Google Scholar]
- Mauro A, Bulfone A, Turco E, Schiffer D. Coexpression of platelet-derived growth factor (PDGF) B chain and PDGF B-type receptor in human gliomas. Childs Nerv Syst. 1991;7:432–436. doi: 10.1007/BF00263184. [DOI] [PubMed] [Google Scholar]
- Takahashi J A, Mori H, Fukumoto M, et al. Gene expression of fibroblast growth factors in human gliomas and meningiomas: demonstration of cellular source of basic fibroblast growth factor mRNA and peptide in tumor tissues. Proc Natl Acad Sci U S A. 1990;87:5710–5714. doi: 10.1073/pnas.87.15.5710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shu J, Lee J H, Harwalkar J A, et al. Adenovirus-mediated gene transfer of dominant negative Ha-Ras inhibits proliferation of primary meningioma cells. Neurosurgery. 1999;44:579–587. discussion 587–588. doi: 10.1097/00006123-199903000-00080. [DOI] [PubMed] [Google Scholar]
- Black P, Carroll R, Zhang J. The molecular biology of hormone and growth factor receptors in meningiomas. Acta Neurochir Suppl. 1996;65:50–53. doi: 10.1007/978-3-7091-9450-8_15. [DOI] [PubMed] [Google Scholar]
- Sanson M, Cornu P. Biology of meningiomas. Acta Neurochir (Wien) 2000;142:493–505. doi: 10.1007/s007010050462. [DOI] [PubMed] [Google Scholar]
- Maxwell M, Galanopoulos T, Neville-Golden J, Antoniades H N. Expression of androgen and progesterone receptors in primary human meningiomas. J Neurosurg. 1993;78:456–462. doi: 10.3171/jns.1993.78.3.0456. [DOI] [PubMed] [Google Scholar]
- Horsfall D J, Goldsmith K G, Ricciardelli C, et al. Steroid hormone and epidermal growth factor receptors in meningiomas. Aust N Z J Surg. 1989;59:881–888. doi: 10.1111/j.1445-2197.1989.tb07033.x. [DOI] [PubMed] [Google Scholar]
- Hsu D W, Efird J T, Hedley-Whyte E T. Progesterone and estrogen receptors in meningiomas: prognostic considerations. J Neurosurg. 1997;86:113–120. doi: 10.3171/jns.1997.86.1.0113. [DOI] [PubMed] [Google Scholar]
- Grunberg S M, Weiss M H, Russell C A, et al. Long-term administration of mifepristone (RU486): clinical tolerance during extended treatment of meningioma. Cancer Invest. 2006;24:727–733. doi: 10.1080/07357900601062339. [DOI] [PubMed] [Google Scholar]
- Ragel B, Jensen R L. New approaches for the treatment of refractory meningiomas. Cancer Control. 2003;10:148–158. doi: 10.1177/107327480301000206. [DOI] [PubMed] [Google Scholar]
- Beschet I, Brunon J, Scoazec J Y, Mosnier J F. Expression of beta1 and beta4 integrins in normal arachnoid membrane and meningiomas. Cancer. 1999;86:2649–2658. [PubMed] [Google Scholar]
- Das A, Tan W L, Smith D R. Expression of extracellular matrix markers in benign meningiomas. Neuropathology. 2003;23:275–281. doi: 10.1046/j.1440-1789.2003.00512.x. [DOI] [PubMed] [Google Scholar]
- Rempel S A, Ge S, Gutierrez J A. SPARC: a potential diagnostic marker of invasive meningiomas. Clin Cancer Res. 1999;5:237–241. [PubMed] [Google Scholar]
- Herman M P, Sukhova G K, Kisiel W, et al. Tissue factor pathway inhibitor-2 is a novel inhibitor of matrix metalloproteinases with implications for atherosclerosis. J Clin Invest. 2001;107:1117–1126. doi: 10.1172/JCI10403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wen P Y, Drappatz J. Novel therapies for meningiomas. Expert Rev Neurother. 2006;6:1447–1464. doi: 10.1586/14737175.6.10.1447. [DOI] [PubMed] [Google Scholar]
- Kondraganti S, Gondi C S, Gujrati M, et al. Restoration of tissue factor pathway inhibitor inhibits invasion and tumor growth in vitro and in vivo in a malignant meningioma cell line. Int J Oncol. 2006;29:25–32. [PMC free article] [PubMed] [Google Scholar]
- Kondraganti S, Gondi C S, McCutcheon I, et al. RNAi-mediated downregulation of urokinase plasminogen activator and its receptor in human meningioma cells inhibits tumor invasion and growth. Int J Oncol. 2006;28:1353–1360. [PMC free article] [PubMed] [Google Scholar]
- Bostrom J, Cobbers J M, Wolter M, et al. Mutation of the PTEN (MMAC1) tumor suppressor gene in a subset of glioblastomas but not in meningiomas with loss of chromosome arm 10q. Cancer Res. 1998;58:29–33. [PubMed] [Google Scholar]
- Shrivastava R K, Segal S, Camins M B, Sen C, Post K D. Harvey Cushing's Meningiomas text and the historical origin of resectability criteria for the anterior one third of the superior sagittal sinus. J Neurosurg. 2003;99:787–791. doi: 10.3171/jns.2003.99.4.0787. [DOI] [PubMed] [Google Scholar]
- Mark J, Levan G, Mitelman F. Identification by fluorescence of the G chromosome lost in human meningomas. Hereditas. 1972;71:163–168. doi: 10.1111/j.1601-5223.1972.tb01017.x. [DOI] [PubMed] [Google Scholar]
- Singer H, Zang K D. Cytological and cytogenetical studies on brain tumors: I. The chromosome aberrations of human meningiomas [in German] Humangenetik. 1970;9:172–184. doi: 10.1007/BF00278933. [DOI] [PubMed] [Google Scholar]
- Zankl H, Seidel H, Zang K D. Cytological and cytogenetical studies on brain tumors: V. Preferential loss of sex chromosomes in human meningiomas. Humangenetik. 1975;27:119–128. [PubMed] [Google Scholar]
- Zankl H, Singer H, Zang K D. Cytological and cytogenetical studies on brain tumors: II. Hyperdiploidy, a rare event in human primary meningiomas. Humangenetik. 1971;11:253–257. doi: 10.1007/BF00274746. [DOI] [PubMed] [Google Scholar]
- Zankl H, Weiss A F, Zang K D. Cytological and cytogenetical studies on brain tumors: VI. No evidence for a translocation in 22-monosomic meningiomas. Humangenetik. 1975;30:343–348. doi: 10.1007/BF00275149. [DOI] [PubMed] [Google Scholar]
- Zankl H, Zang K D. Cytological and cytogenetical studies on brain tumors: III. Ph1-like chromosomes in human meningiomas. Humangenetik. 1971;12:42–49. doi: 10.1007/BF00291031. [DOI] [PubMed] [Google Scholar]
- Zankl H, Zang K D. Cytological and cytogenetical studies on brain tumors: IV. Identification of the missing G chromosome in human meningiomas as no. 22 by fluorescence technique. Humangenetik. 1972;14:167–169. doi: 10.1007/BF00273305. [DOI] [PubMed] [Google Scholar]
- Seizinger B R, Rouleau G, Lane A H, et al. DNA linkage analysis in Von Recklinghausen neurofibromatosis. J Med Genet. 1987;24:529–530. doi: 10.1136/jmg.24.9.529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seizinger B R, Rouleau G, Ozelius L J, et al. Common pathogenetic mechanism for three tumor types in bilateral acoustic neurofibromatosis. Science. 1987;236:317–319. doi: 10.1126/science.3105060. [DOI] [PubMed] [Google Scholar]
- Seizinger B R, Rouleau G A, Ozelius L J, et al. Genetic linkage of von Recklinghausen neurofibromatosis to the nerve growth factor receptor gene. Cell. 1987;49:589–594. doi: 10.1016/0092-8674(87)90534-4. [DOI] [PubMed] [Google Scholar]
- Evans J J, Jeun S S, Lee J H, et al. Molecular alterations in the neurofibromatosis type 2 gene and its protein rarely occurring in meningothelial meningiomas. J Neurosurg. 2001;94:111–117. doi: 10.3171/jns.2001.94.1.0111. [DOI] [PubMed] [Google Scholar]
- De Vitis L R, Tedde A, Vitelli F, et al. Screening for mutations in the neurofibromatosis type 2 (NF2) gene in sporadic meningiomas. Hum Genet. 1996;97:632–637. doi: 10.1007/BF02281874. [DOI] [PubMed] [Google Scholar]
- De Vitis L R, Tedde A, Vitelli F, et al. Analysis of the neurofibromatosis type 2 gene in different human tumors of neuroectodermal origin. Hum Genet. 1996;97:638–641. doi: 10.1007/BF02281875. [DOI] [PubMed] [Google Scholar]
- Messerini L, Vitelli F, De Vitis L R, et al. Microsatellite instability in sporadic mucinous colorectal carcinomas: relationship to clinico-pathological variables. J Pathol. 1997;182:380–384. doi: 10.1002/(SICI)1096-9896(199708)182:4<380::AID-PATH871>3.0.CO;2-U. [DOI] [PubMed] [Google Scholar]
- Papi L, De Vitis L R, Vitelli F, et al. Somatic mutations in the neurofibromatosis type 2 gene in sporadic meningiomas. Hum Genet. 1995;95:347–351. doi: 10.1007/BF00225206. [DOI] [PubMed] [Google Scholar]
- von Deimling A, Kraus J A, Stangl A P, et al. Evidence for subarachnoid spread in the development of multiple meningiomas. Brain Pathol. 1995;5:11–14. doi: 10.1111/j.1750-3639.1995.tb00571.x. [DOI] [PubMed] [Google Scholar]
- Wellenreuther R, Kraus J A, Lenartz D, et al. Analysis of the neurofibromatosis 2 gene reveals molecular variants of meningioma. Am J Pathol. 1995;146:827–832. [PMC free article] [PubMed] [Google Scholar]
- Ruttledge M H, Sarrazin J, Rangaratnam S, et al. Evidence for the complete inactivation of the NF2 gene in the majority of sporadic meningiomas. Nat Genet. 1994;6:180–184. doi: 10.1038/ng0294-180. [DOI] [PubMed] [Google Scholar]
- Watkins D, Ruttledge M H, Sarrazin J, et al. Loss of heterozygosity on chromosome 22 in human gliomas does not inactivate the neurofibromatosis type 2 gene. Cancer Genet Cytogenet. 1996;92:73–78. doi: 10.1016/s0165-4608(96)00149-5. [DOI] [PubMed] [Google Scholar]
- Bigner S H, Mark J, Mahaley M S, Bigner D D. Patterns of the early, gross chromosomal changes in malignant human gliomas. Hereditas. 1984;101:103–113. doi: 10.1111/j.1601-5223.1984.tb00455.x. [DOI] [PubMed] [Google Scholar]
- Perry A, Cai D X, Scheithauer B W, et al. Merlin, DAL-1, and progesterone receptor expression in clinicopathologic subsets of meningioma: a correlative immunohistochemical study of 175 cases. J Neuropathol Exp Neurol. 2000;59:872–879. doi: 10.1093/jnen/59.10.872. [DOI] [PubMed] [Google Scholar]
- Gutmann D H, Donahoe J, Perry A, et al. Loss of DAL-1, a protein 4.1-related tumor suppressor, is an important early event in the pathogenesis of meningiomas. Hum Mol Genet. 2000;9:1495–1500. doi: 10.1093/hmg/9.10.1495. [DOI] [PubMed] [Google Scholar]
- Shoshan Y, Chernova O, Juen S S, et al. Radiation-induced meningioma: a distinct molecular genetic pattern? J Neuropathol Exp Neurol. 2000;59:614–620. doi: 10.1093/jnen/59.7.614. [DOI] [PubMed] [Google Scholar]
- Bello M J, de Campos J M, Kusak M E, et al. Allelic loss at 1p is associated with tumor progression of meningiomas. Genes Chromosomes Cancer. 1994;9:296–298. doi: 10.1002/gcc.2870090411. [DOI] [PubMed] [Google Scholar]
- Bello M J, Leone P E, Nebreda P, et al. Allelic status of chromosome 1 in neoplasms of the nervous system. Cancer Genet Cytogenet. 1995;83:160–164. doi: 10.1016/0165-4608(95)00064-v. [DOI] [PubMed] [Google Scholar]
- Bostrom J, Meyer-Puttlitz B, Wolter M, et al. Alterations of the tumor suppressor genes CDKN2A (p16(INK4a)), p14(ARF), CDKN2B (p15(INK4b)), and CDKN2C (p18(INK4c)) in atypical and anaplastic meningiomas. Am J Pathol. 2001;159:661–669. doi: 10.1016/S0002-9440(10)61737-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cai D X, Banerjee R, Scheithauer B W, et al. Chromosome 1p and 14q FISH analysis in clinicopathologic subsets of meningioma: diagnostic and prognostic implications. J Neuropathol Exp Neurol. 2001;60:628–636. doi: 10.1093/jnen/60.6.628. [DOI] [PubMed] [Google Scholar]
- Santarius T, Kirsch M, Nikas D C, Imitola J, Black P M. Molecular analysis of alterations of the p18INK4c gene in human meningiomas. Neuropathol Appl Neurobiol. 2000;26:67–75. doi: 10.1046/j.1365-2990.2000.00219.x. [DOI] [PubMed] [Google Scholar]
- Alonso M E, Bello M J, Lomas J, et al. Absence of mutation of the p73 gene in astrocytic neoplasms. Int J Oncol. 2001;19:609–612. doi: 10.3892/ijo.19.3.609. [DOI] [PubMed] [Google Scholar]
- Lomas J, Bello M J, Arjona D, et al. Analysis of p73 gene in meningiomas with deletion at 1p. Cancer Genet Cytogenet. 2001;129:88–91. doi: 10.1016/s0165-4608(01)00430-7. [DOI] [PubMed] [Google Scholar]
- Nozaki M, Tada M, Kashiwazaki H, et al. p73 is not mutated in meningiomas as determined with a functional yeast assay but p73 expression increases with tumor grade. Brain Pathol. 2001;11:296–305. doi: 10.1111/j.1750-3639.2001.tb00400.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sulman E P, Dumanski J P, White P S, et al. Identification of a consistent region of allelic loss on 1p32 in meningiomas: correlation with increased morbidity. Cancer Res. 1998;58:3226–3230. [PubMed] [Google Scholar]
- Bostrom J, Muhlbauer A, Reifenberger G. Deletion mapping of the short arm of chromosome 1 identifies a common region of deletion distal to D1S496 in human meningiomas. Acta Neuropathol (Berl) 1997;94:479–485. doi: 10.1007/s004010050736. [DOI] [PubMed] [Google Scholar]
- Perry A, Banerjee R, Lohse C M, Kleinschmidt-DeMasters B K, Scheithauer B W. A role for chromosome 9p21 deletions in the malignant progression of meningiomas and the prognosis of anaplastic meningiomas. Brain Pathol. 2002;12:183–190. doi: 10.1111/j.1750-3639.2002.tb00433.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tran T P, Titus-Ernstoff L, Perry A E, Ernstoff M S, Newsham I F. Alteration of chromosome 9p21 and/or p16 in benign and dysplastic nevi suggests a role in early melanoma progression (United States) Cancer Causes Control. 2002;13:675–682. doi: 10.1023/a:1019599629895. [DOI] [PubMed] [Google Scholar]
- Cerda-Nicolas M, Lopez-Gines C, Gil-Benso R, et al. Solitary fibrous tumor of the orbit: morphological, cytogenetic and molecular features. Neuropathology. 2006;26:557–563. doi: 10.1111/j.1440-1789.2006.00726.x. [DOI] [PubMed] [Google Scholar]
- Korshunov A, Shishkina L, Golanov A. Immunohistochemical analysis of p16INK4a, p14ARF, p18INK4c, p21CIP1, p27KIP1 and p73 expression in 271 meningiomas correlation with tumor grade and clinical outcome. Int J Cancer. 2003;104:728–734. doi: 10.1002/ijc.11013. [DOI] [PubMed] [Google Scholar]
- Perry A, Gutmann D H, Reifenberger G. Molecular pathogenesis of meningiomas. J Neurooncol. 2004;70:183–202. doi: 10.1007/s11060-004-2749-0. [DOI] [PubMed] [Google Scholar]
- Simon M, Park T W, Koster G, et al. Alterations of INK4a(p16-p14ARF)/INK4b(p15) expression and telomerase activation in meningioma progression. J Neurooncol. 2001;55:149–158. doi: 10.1023/a:1013863630293. [DOI] [PubMed] [Google Scholar]
- Rempel S A, Schwechheimer K, Davis R L, Cavenee W K, Rosenblum M L. Loss of heterozygosity for loci on chromosome 10 is associated with morphologically malignant meningioma progression. Cancer Res. 1993;53(10, Suppl):2386–2392. [PubMed] [Google Scholar]
- Weber R G, Bostrom J, Wolter M, et al. Analysis of genomic alterations in benign, atypical, and anaplastic meningiomas: toward a genetic model of meningioma progression. Proc Natl Acad Sci U S A. 1997;94:14719–14724. doi: 10.1073/pnas.94.26.14719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mihaila D, Jankowski M, Gutierrez J A, et al. Meningiomas: loss of heterozygosity on chromosome 10 and marker-specific correlations with grade, recurrence, and survival. Clin Cancer Res. 2003;9:4443–4451. [PubMed] [Google Scholar]
- Joachim T, Ram Z, Rappaport Z H, et al. Comparative analysis of the NF2, TP53, PTEN, KRAS, NRAS and HRAS genes in sporadic and radiation-induced human meningiomas. Int J Cancer. 2001;94:218–221. doi: 10.1002/ijc.1467. [DOI] [PubMed] [Google Scholar]
- Dirven C M, Grill J, Lamfers M L, et al. Gene therapy for meningioma: improved gene delivery with targeted adenoviruses. J Neurosurg. 2002;97:441–449. doi: 10.3171/jns.2002.97.2.0441. [DOI] [PubMed] [Google Scholar]
- Tse J Y, Ng H K, Lo K W, et al. Analysis of cell cycle regulators: p16INK4A, pRb, and CDK4 in low- and high-grade meningiomas. Hum Pathol. 1998;29:1200–1207. doi: 10.1016/s0046-8177(98)90246-5. [DOI] [PubMed] [Google Scholar]
- Tse J Y, Ng H K, Lau K M, et al. Loss of heterozygosity of chromosome 14q in low- and high-grade meningiomas. Hum Pathol. 1997;28:779–785. doi: 10.1016/s0046-8177(97)90149-0. [DOI] [PubMed] [Google Scholar]
- Maillo A, Orfao A, Sayagues J M, et al. New classification scheme for the prognostic stratification of meningioma on the basis of chromosome 14 abnormalities, patient age, and tumor histopathology. J Clin Oncol. 2003;21:3285–3295. doi: 10.1200/JCO.2003.07.156. [DOI] [PubMed] [Google Scholar]
- Lusis E A, Watson M A, Chicoine M R, et al. Integrative genomic analysis identifies NDRG2 as a candidate tumor suppressor gene frequently inactivated in clinically aggressive meningioma. Cancer Res. 2005;65:7121–7126. doi: 10.1158/0008-5472.CAN-05-0043. [DOI] [PubMed] [Google Scholar]
- Ohgaki H, Eibl R H, Schwab M, et al. Mutations of the p53 tumor suppressor gene in neoplasms of the human nervous system. Mol Carcinog. 1993;8:74–80. doi: 10.1002/mc.2940080203. [DOI] [PubMed] [Google Scholar]
- Ohgaki H, Eibl R H, Wiestler O D, et al. p53 mutations in nonastrocytic human brain tumors. Cancer Res. 1991;51:6202–6205. [PubMed] [Google Scholar]
- Perry A, Stafford S L, Scheithauer B W, Suman V J, Lohse C M. The prognostic significance of MIB-1, p53, and DNA flow cytometry in completely resected primary meningiomas. Cancer. 1998;82:2262–2269. [PubMed] [Google Scholar]
- Karamitopoulou E, Perentes E, Tolnay M, Probst A. Prognostic significance of MIB-1, p53, and bcl-2 immunoreactivity in meningiomas. Hum Pathol. 1998;29:140–145. doi: 10.1016/s0046-8177(98)90224-6. [DOI] [PubMed] [Google Scholar]
- Büschges R, Ichimura K, Weber R G, Reifenberger G, Collins V P. Allelic gain and amplification on the long arm of chromosome 17 in anaplastic meningiomas. Brain Pathol. 2002;12:145–153. doi: 10.1111/j.1750-3639.2002.tb00429.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Büschges R, Bostrom J, Wolter M, et al. Analysis of human meningiomas for aberrations of the MADH2, MADH4, APM-1 and DCC tumor suppressor genes on the long arm of chromosome 18. Int J Cancer. 2001;92:551–554. doi: 10.1002/ijc.1219. [DOI] [PubMed] [Google Scholar]
- Menon A G, Rutter J L, von Sattel J P, et al. Frequent loss of chromosome 14 in atypical and malignant meningioma: identification of a putative “tumor progression” locus. Oncogene. 1997;14:611–616. doi: 10.1038/sj.onc.1200853. [DOI] [PubMed] [Google Scholar]
- Kazumoto K, Tamura M, Hoshino H, Yuasa Y. Enhanced expression of the sis and c-myc oncogenes in human meningiomas. J Neurosurg. 1990;72:786–791. doi: 10.3171/jns.1990.72.5.0786. [DOI] [PubMed] [Google Scholar]
- Tanaka K, Sato C, Maeda Y, et al. Establishment of a human malignant meningioma cell line with amplified c-myc oncogene. Cancer. 1989;64:2243–2249. doi: 10.1002/1097-0142(19891201)64:11<2243::aid-cncr2820641110>3.0.co;2-s. [DOI] [PubMed] [Google Scholar]
- Shiurba R A, Eng L F, Vogel H, et al. Epidermal growth factor receptor in meningiomas is expressed predominantly on endothelial cells. Cancer. 1988;62:2139–2144. doi: 10.1002/1097-0142(19881115)62:10<2139::aid-cncr2820621013>3.0.co;2-g. [DOI] [PubMed] [Google Scholar]
- Langford L A, Piatyszek M A, Xu R, et al. Telomerase activity in ordinary meningiomas predicts poor outcome. Hum Pathol. 1997;28:416–420. doi: 10.1016/s0046-8177(97)90029-0. [DOI] [PubMed] [Google Scholar]
- Cabuy E, de Ridder L. Telomerase activity and expression of telomerase reverse transcriptase correlated with cell proliferation in meningiomas and malignant brain tumors in vivo. Virchows Arch. 2001;439:176–184. doi: 10.1007/s004280100466. [DOI] [PubMed] [Google Scholar]
- Folkman J, Ingber D. Inhibition of angiogenesis. Semin Cancer Biol. 1992;3:89–96. [PubMed] [Google Scholar]
- Kaba S E, DeMonte F, Bruner J M, et al. The treatment of recurrent unresectable and malignant meningiomas with interferon alpha-2B. Neurosurgery. 1997;40:271–275. doi: 10.1097/00006123-199702000-00007. [DOI] [PubMed] [Google Scholar]
- Zhang Z J, Muhr C, Wang J L. Interferon-alpha inhibits the DNA synthesis induced by PDGF and EGF in cultured meningioma cells. Anticancer Res. 1996;16:717–723. [PubMed] [Google Scholar]
- Zhang Z J, Wang J L, Muhr C, Smits A. Synergistic inhibitory effects of interferon-alpha and 5-fluorouracil in meningioma cells in vitro. Cancer Lett. 1996;100(1–2):99–105. doi: 10.1016/0304-3835(95)04076-5. [DOI] [PubMed] [Google Scholar]
- Wang J L, Zhang Z J, Hartman M, et al. Detection of TP53 gene mutation in human meningiomas: a study using immunohistochemistry, polymerase chain reaction/single-strand conformation polymorphism and DNA sequencing techniques on paraffin-embedded samples. Int J Cancer. 1995;64:223–228. doi: 10.1002/ijc.2910640402. [DOI] [PubMed] [Google Scholar]
- Muhr C, Gudjonsson O, Lilja A, et al. Meningioma treated with interferon-alpha, evaluated with [(11)C]-L-methionine positron emission tomography. Clin Cancer Res. 2001;7:2269–2276. [PubMed] [Google Scholar]
- Bek E L, McMillen M A, Scott P, Angus L D, Shaftan G W. The effect of diabetes on endothelin, interleukin-8 and vascular endothelial growth factor-mediated angiogenesis in rats. Clin Sci (Lond) 2002;103(suppl 48):424S–429S. doi: 10.1042/CS103S424S. [DOI] [PubMed] [Google Scholar]
- Bek E L, McMillen M A. Endothelins are angiogenic. J Cardiovasc Pharmacol. 2000;36(suppl 1):S135–S139. doi: 10.1097/00005344-200036051-00043. [DOI] [PubMed] [Google Scholar]
- Harland S P, Kuc R E, Pickard J D, Davenport A P. Expression of endothelin(A) receptors in human gliomas and meningiomas, with high affinity for the selective antagonist PD156707. Neurosurgery. 1998;43:890–898. discussion 898–899. doi: 10.1097/00006123-199810000-00097. [DOI] [PubMed] [Google Scholar]
- Harland S P, Kuc R E, Pickard J D, Davenport A P. Characterization of endothelin receptors in human brain cortex, gliomas, and meningiomas. J Cardiovasc Pharmacol. 1995;26(suppl 3):S408–S411. [PubMed] [Google Scholar]
- Salhia B, Rutka J T, Lingwood C, Nutikka A, Furth W R Van. The treatment of malignant meningioma with verotoxin. Neoplasia. 2002;4:304–311. doi: 10.1038/sj.neo.7900243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lawrence J H, Tobias C A, Linfoot J A, et al. Successful treatment of acromegaly: metabolic and clinical studies in 145 patients. J Clin Endocrinol Metab. 1970;31:180–198. doi: 10.1210/jcem-31-2-180. [DOI] [PubMed] [Google Scholar]
- McCutcheon I E, Flyvbjerg A, Hill H, et al. Antitumor activity of the growth hormone receptor antagonist pegvisomant against human meningiomas in nude mice. J Neurosurg. 2001;94:487–492. doi: 10.3171/jns.2001.94.3.0487. [DOI] [PubMed] [Google Scholar]
- Friend K E, Khandwala H M, Flyvbjerg A, et al. Growth hormone and insulin-like growth factor-I: effects on the growth of glioma cell lines. Growth Horm IGF Res. 2001;11:84–91. doi: 10.1054/ghir.2000.0183. [DOI] [PubMed] [Google Scholar]
- Trainer P J, Drake W M, Katznelson L, et al. Treatment of acromegaly with the growth hormone-receptor antagonist pegvisomant. N Engl J Med. 2000;342:1171–1177. doi: 10.1056/NEJM200004203421604. [DOI] [PubMed] [Google Scholar]
- Jensen R L, Lee Y S, Guijrati M, et al. Inhibition of in vitro meningioma proliferation after growth factor stimulation by calcium channel antagonists: Part II—additional growth factors, growth factor receptor immunohistochemistry, and intracellular calcium measurements. Neurosurgery. 1995;37:937–946. discussion 946–947. doi: 10.1227/00006123-199511000-00013. [DOI] [PubMed] [Google Scholar]
- Jensen R L, Origitano T C, Lee Y S, Weber M, Wurster R D. In vitro growth inhibition of growth factor-stimulated meningioma cells by calcium channel antagonists. Neurosurgery. 1995;36:365–373. discussion 373–374. doi: 10.1227/00006123-199502000-00017. [DOI] [PubMed] [Google Scholar]
- Jensen R L, Petr M, Wurster R D. Calcium channel antagonist effect on in vitro meningioma signal transduction pathways after growth factor stimulation. Neurosurgery. 2000;46:692–702. discussion 702–703. doi: 10.1097/00006123-200003000-00032. [DOI] [PubMed] [Google Scholar]
- Bowles A P, Jr, Pantazis C G, Wansley W. Use of verapamil to enhance the antiproliferative activity of BCNU in human glioma cells: an in vitro and in vivo study. J Neurosurg. 1990;73:248–253. doi: 10.3171/jns.1990.73.2.0248. [DOI] [PubMed] [Google Scholar]
- Cano-Gauci D F, Seibert F S, Safa A R, Riordan J R. Selection and characterization of verapamil-resistant multidrug resistant cells. Biochem Biophys Res Commun. 1995;209:497–505. doi: 10.1006/bbrc.1995.1529. [DOI] [PubMed] [Google Scholar]
- Cano-Gauci D F, Riordan J R. Action of calcium antagonists on multidrug resistant cells: specific cytotoxicity independent of increased cancer drug accumulation. Biochem Pharmacol. 1987;36:2115–2123. doi: 10.1016/0006-2952(87)90139-0. [DOI] [PubMed] [Google Scholar]
- de Keizer R J, Smit J W. Mifepristone treatment in patients with surgically incurable sphenoid-ridge meningioma: a long-term follow-up. Eye. 2004;18:954–958. doi: 10.1038/sj.eye.6701370. [DOI] [PubMed] [Google Scholar]
- Kalamarides M, Niwa-Kawakita M, Leblois H, et al. NF2 gene inactivation in arachnoidal cells is rate-limiting for meningioma development in the mouse. Genes Dev. 2002;16:1060–1065. doi: 10.1101/gad.226302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chauvet A E, Kesava P P, Goh C S, Badie B. Selective intraarterial gene delivery into a canine meningioma. J Neurosurg. 1998;88:870–873. doi: 10.3171/jns.1998.88.5.0870. [DOI] [PubMed] [Google Scholar]
- Ikeda H, Adachi-Usami E, Saeki M, Takeda N, Kimura T. Electrophysiological studies on light damage in the mouse retina after sodium iodate injection. Ophthalmologica. 1994;208:220–225. doi: 10.1159/000310493. [DOI] [PubMed] [Google Scholar]