Abstract
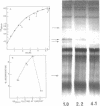
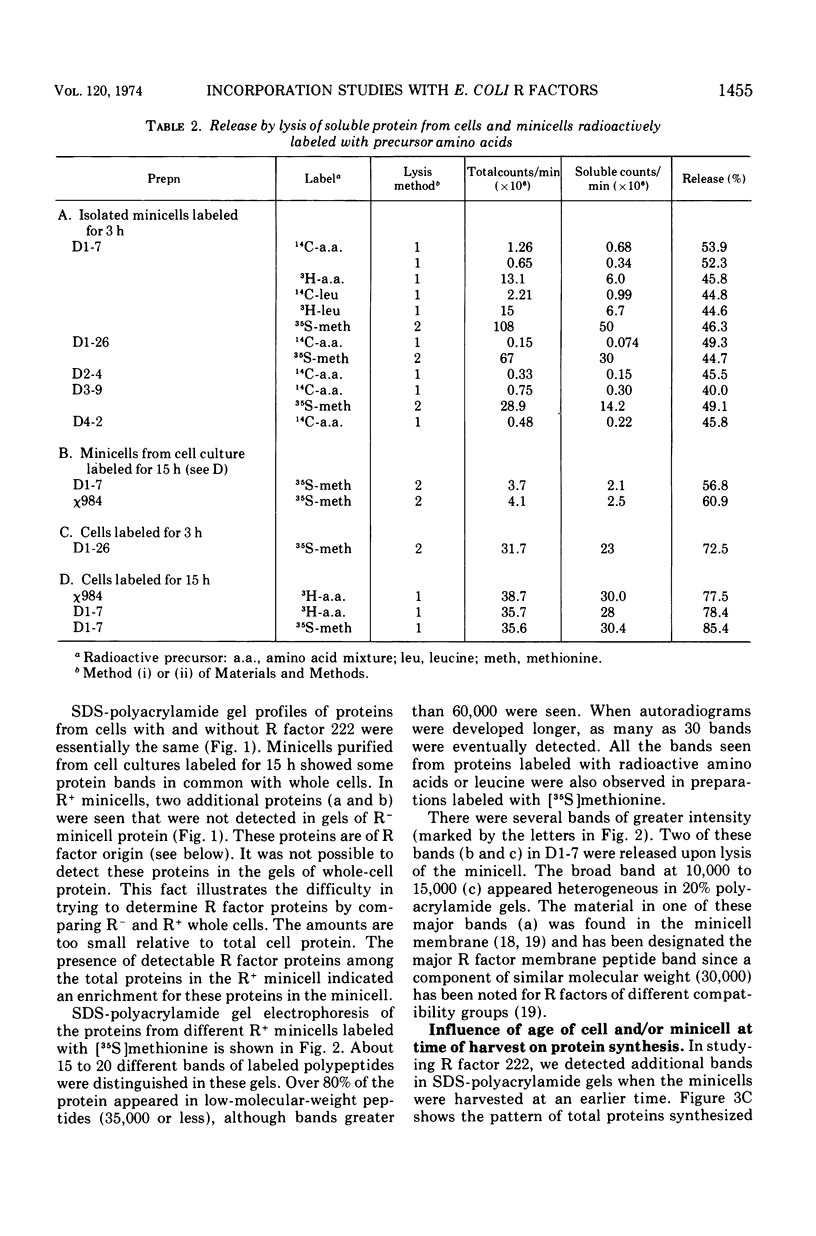
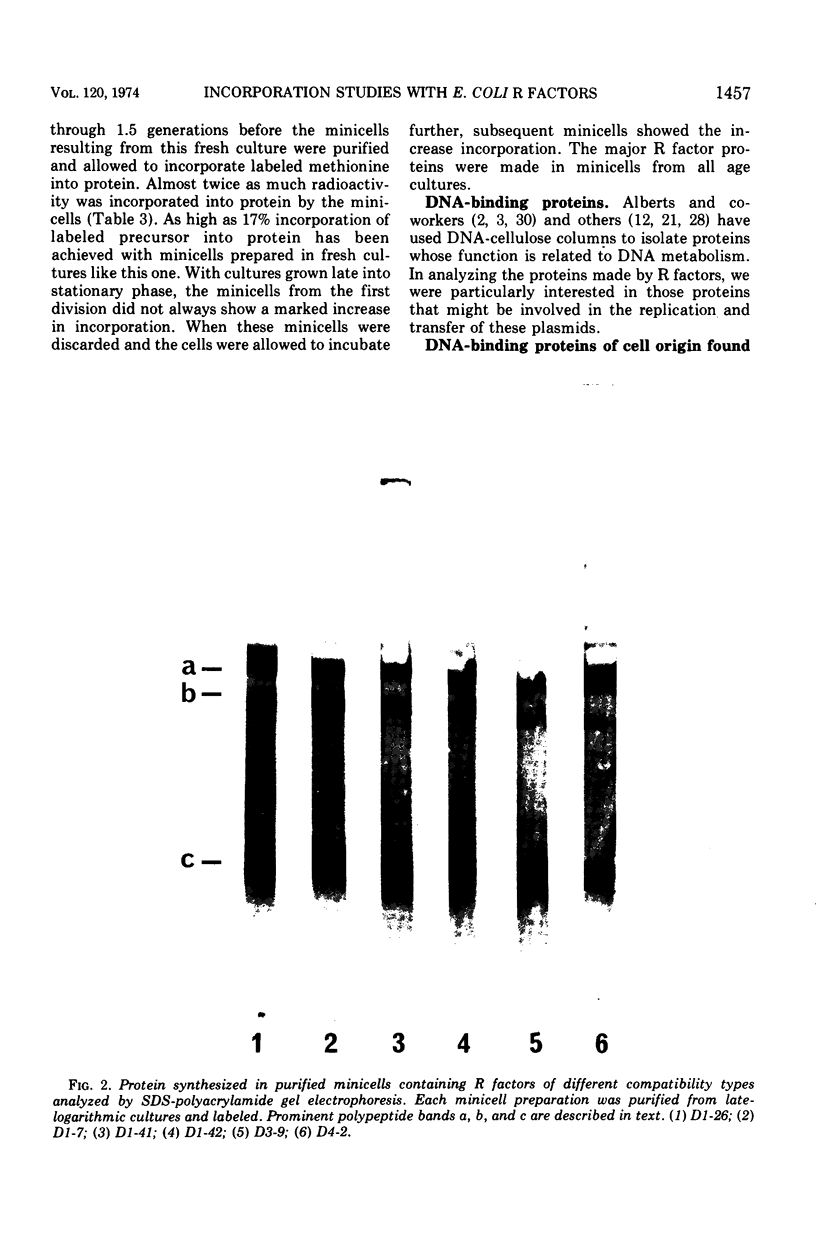
Analysis of the protein synthesized by Escherichia coli minicells containing R factors demonstrated a variety of low- and high-molecular-weight polypeptides in sodium dodecyl sulfate (SDS)-polyacrylamide gels. Only half of this protein was released into a soluble fraction on lysis of these minicells. The other half remained associated with the minicell envelope. The efficiency of precursor incorporation into protein and the kinds of proteins synthesized changed with the age of the minicells at the time of harvest. About 1 to 2% of the soluble R factor-coded protein bound to calf thymus, E. coli, or R factor DNA-cellulose. Although most of these proteins were excluded from Sephadex G-100 columns, they migrated chiefly as low-molecular-weight-polypeptides (13,000 to 15,000) in SDS-polyacrylamide gels. Additional DNA-binding proteins that appeared to be higher-molecular-weight peptides were noted in extracts from younger minicells. At least one protein, identified as an SDS band, appeared to bind selectively to R factor DNA-cellulose. Minicells with R factors also contained DNA-binding proteins of cell origin, including the core RNA polymerase. No such binding proteins were found in R− minicells. These studies suggest that: (i) R factors code for proteins that may be involved in their own DNA metabolism; (ii) R factor DNA-binding proteins may be associated with larger host cell DNA-binding proteins or subunits of larger R factor proteins; and (iii) the age of the minicell influences the extent of protein synthesis and the kinds of proteins synthesized by R factors in minicells.
Full text
PDF










Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Adler H. I., Fisher W. D., Cohen A., Hardigree A. A. MINIATURE escherichia coli CELLS DEFICIENT IN DNA. Proc Natl Acad Sci U S A. 1967 Feb;57(2):321–326. doi: 10.1073/pnas.57.2.321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alberts B. M., Amodio F. J., Jenkins M., Gutmann E. D., Ferris F. L. Studies with DNA-cellulose chromatography. I. DNA-binding proteins from Escherichia coli. Cold Spring Harb Symp Quant Biol. 1968;33:289–305. doi: 10.1101/sqb.1968.033.01.033. [DOI] [PubMed] [Google Scholar]
- Chabbert Y. A., Scavizzi M. R., Witchitz J. L., Gerbaud G. R., Bouanchaud D. H. Incompatibility groups and the classification of fi - resistance factors. J Bacteriol. 1972 Nov;112(2):666–675. doi: 10.1128/jb.112.2.666-675.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Datta N., Hedges R. W. Compatibility groups among fi - R factors. Nature. 1971 Nov 26;234(5326):222–223. doi: 10.1038/234222a0. [DOI] [PubMed] [Google Scholar]
- Davies J. E., Rownd R. Transmissible multiple drug resistance in Enterobacteriaceae. Science. 1972 May 19;176(4036):758–768. doi: 10.1126/science.176.4036.758. [DOI] [PubMed] [Google Scholar]
- Fralick J. A., Fisher W. D., Adler H. I. Polyuridylic acid-directed phenylalanine incorporation in minicell extracts. J Bacteriol. 1969 Aug;99(2):621–622. doi: 10.1128/jb.99.2.621-622.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frazer A. C., Curtiss R., 3rd Derepression of anthranilate synthase in purified minicells of Escherichia coli containing the Col-trp plasmid. J Bacteriol. 1973 Aug;115(2):615–622. doi: 10.1128/jb.115.2.615-622.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hedges R. W., Datta N. R124, an fi R factor of a new compatibility class. J Gen Microbiol. 1972 Jul;71(2):403–405. doi: 10.1099/00221287-71-2-403. [DOI] [PubMed] [Google Scholar]
- Inselburg J. R factor deoxyribonucleic acid in chromosomeless progeny of Escherichia coli. J Bacteriol. 1971 Feb;105(2):620–628. doi: 10.1128/jb.105.2.620-628.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kass L. R., Yarmolinsky M. B. Segregation of functional sex factor into minicells. Proc Natl Acad Sci U S A. 1970 Jul;66(3):815–822. doi: 10.1073/pnas.66.3.815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Katz S. E., Hecht N. B. A rapid technique to resolve and partially purify multiple DNA polymerases from a crude extract. Anal Biochem. 1973 Jun;53(2):441–444. doi: 10.1016/0003-2697(73)90091-2. [DOI] [PubMed] [Google Scholar]
- Kool A. J., van Zeben M. S., Nijkamp H. J. Identification of messenger ribonucleic acids and proteins synthesized by the bacteriocinogenic factor Clo DF13 in purified minicells of Escherichia coli. J Bacteriol. 1974 Apr;118(1):213–224. doi: 10.1128/jb.118.1.213-224.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LENNOX E. S. Transduction of linked genetic characters of the host by bacteriophage P1. Virology. 1955 Jul;1(2):190–206. doi: 10.1016/0042-6822(55)90016-7. [DOI] [PubMed] [Google Scholar]
- Levy S. B., McMurry L. Detection of an inducible membrane protein associated with R-factor-mediated tetracycline resistance. Biochem Biophys Res Commun. 1974 Feb 27;56(4):1060–1068. doi: 10.1016/s0006-291x(74)80296-2. [DOI] [PubMed] [Google Scholar]
- Levy S. B., McMurry L., Palmer E. R factor proteins synthesized in Escherichia coli minicells: membrane-associated R factor proteins. J Bacteriol. 1974 Dec;120(3):1464–1471. doi: 10.1128/jb.120.3.1464-1471.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levy S. B., Norman P. Segregation of transferable R factors into Escherichia coli minicells. Nature. 1970 Aug 8;227(5258):606–607. doi: 10.1038/227606a0. [DOI] [PubMed] [Google Scholar]
- Levy S. B. Physical and functional characteristics of R-factor deoxyribonucleic acid segregated into Escherichia coli minicells. J Bacteriol. 1971 Oct;108(1):300–308. doi: 10.1128/jb.108.1.300-308.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levy S. B. Resistance of minicells to penicillin lysis: a method of obtaining large quantities of purified minicells. J Bacteriol. 1970 Sep;103(3):836–839. doi: 10.1128/jb.103.3.836-839.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levy S. B. The problems of drug-resistant pathogenic bacteria. Studies on R factors segregated into E. coli minicells. Ann N Y Acad Sci. 1971 Jun 11;182:217–225. doi: 10.1111/j.1749-6632.1971.tb30658.x. [DOI] [PubMed] [Google Scholar]
- Litman R. M. A deoxyribonucleic acid polymerase from Micrococcus luteus (Micrococcus lysodeikticus) isolated on deoxyribonucleic acid-cellulose. J Biol Chem. 1968 Dec 10;243(23):6222–6233. [PubMed] [Google Scholar]
- MALAMY M., HORECKER B. L. The localization of alkaline phosphatase in E. coli K12. Biochem Biophys Res Commun. 1961 Jun 2;5:104–108. doi: 10.1016/0006-291x(61)90020-1. [DOI] [PubMed] [Google Scholar]
- Otsubo E., Nishimura Y., Hirota Y. Transfer-defective mutants of sex factors in Escherichia coli. I. Defective mutants and complementation analysis. Genetics. 1970 Feb;64(2):173–188. doi: 10.1093/genetics/64.2.173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prouty W. F., Goldberg A. L. Effects of protease inhibitors on protein breakdown in Escherichia coli. J Biol Chem. 1972 May 25;247(10):3341–3352. [PubMed] [Google Scholar]
- Roozen K. J., Fenwick R. G., Jr, Curtiss R., 3rd Synthesis of ribonucleic acid and protein in plasmid-containing minicells of Escherichia coli K-12. J Bacteriol. 1971 Jul;107(1):21–33. doi: 10.1128/jb.107.1.21-33.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scherzinger E., Litfin F., Jost E. Stimulation of T7 DNA polymerase by a new phage-coded protein. Mol Gen Genet. 1973 Jul 2;123(3):247–262. doi: 10.1007/BF00271243. [DOI] [PubMed] [Google Scholar]
- Schnaitman C. A. Examination of the protein composition of the cell envelope of Escherichia coli by polyacrylamide gel electrophoresis. J Bacteriol. 1970 Nov;104(2):882–889. doi: 10.1128/jb.104.2.882-889.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sigal N., Delius H., Kornberg T., Gefter M. L., Alberts B. A DNA-unwinding protein isolated from Escherichia coli: its interaction with DNA and with DNA polymerases. Proc Natl Acad Sci U S A. 1972 Dec;69(12):3537–3541. doi: 10.1073/pnas.69.12.3537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stevens A. New small polypeptides associated with DNA-dependent RNA polymerase of Escherichia coli after infection with bacteriophage T4. Proc Natl Acad Sci U S A. 1972 Mar;69(3):603–607. doi: 10.1073/pnas.69.3.603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Studier F. W., Maizel J. V., Jr T7-directed protein synthesis. Virology. 1969 Nov;39(3):575–586. doi: 10.1016/0042-6822(69)90105-6. [DOI] [PubMed] [Google Scholar]
- WATANABE T. Infective heredity of multiple drug resistance in bacteria. Bacteriol Rev. 1963 Mar;27:87–115. doi: 10.1128/br.27.1.87-115.1963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weber K., Osborn M. The reliability of molecular weight determinations by dodecyl sulfate-polyacrylamide gel electrophoresis. J Biol Chem. 1969 Aug 25;244(16):4406–4412. [PubMed] [Google Scholar]
- van Embden J., Cohen S. N. Molecular and genetic studies of an R factor system consisting of independent transfer and drug resistance plasmids. J Bacteriol. 1973 Nov;116(2):699–709. doi: 10.1128/jb.116.2.699-709.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]