Abstract
The steroid hormone, estradiol (E2), has numerous targets in the central nervous system, including the hippocampus, which plays a key role in cognition and affective behavior. This review focuses on our evidence from studies in rodents that E2 has diverse mechanisms in the hippocampus for its functional effects E2 has rapid, membrane-mediated effects in the hippocampus to enhance cognitive performance. Administration of E2 to the hippocampus of rats for 10 minutes following training enhances performance in a hippocampus-mediated task. Increased cell firing in the hippocampus occurs within this short time frame. Furthermore, administration of free E2 or an E2 conjugate, E2:bovine serum albumin (BSA), to the hippocampus produces similar performance-enhancing effects in this task, suggesting that E2 has membrane actions in the hippocampus for these effects. Further evidence that E2 has rapid, membrane-mediated effects is that co-administration of E2 and inhibitors of mitogen activated protein kinase (MAPK), rather than intracellular E2 receptors (ERs) or protein synthesis, attenuate the enhancing effects of E2 in this task. Despite these data that demonstrate E2 can have rapid and/or membrane-mediated effects in the hippocampus, there is clear evidence to suggest that intracellular ERs, particularly the β (rather than α) isoform of ERs, may be important targets for E2’s functional effects for hippocampal processes. Administration of ligands that are specific for ERβ, but not ERα, have enhancing effects on hippocampal processes similar to that of E2 (which has similar affinity for ERα and ERβ). These effects are attenuated when ERβ expression is knocked down in transgenic models or with central administration of antisense oligonucleotides. Thus, there may be a convergence of E2’s actions through rapid, membrane-mediated effects and intracellular ERs and in the hippocampus for these functional effects.
Keywords: estradiol, estrogen receptor beta, rapid, membrane, cognition, anxiety
INTRODUCTION
There are many potential mechanism(s) of action by which E2 may influence functions mediated by the hippocampus, such as cognitive and/or affective behaviors. Due to the multiplicity of mechanisms, a comprehensive discussion them all is beyond the scope of this review. One manageable approach to begin to consider divergent mechanisms of E2 is to investigate the two different broad categories of E2 actions, i.e. non-classical actions that are rapid and/or membrane-mediated and classical actions involving binding to intracellular estrogen receptors (ERs). E2 may have membrane actions that involve rapid signaling and/or actions at membrane receptor targets that initiate signal transduction cascades (e.g. MAPK, AKT, CREB). E2 can also have traditional actions through cognate intracellular ERs, of which two subtypes have been characterized (α and β), and bind to the E2 response element (ERE) or the AP-1 binding site. Notably, it may be that E2’s actions involve integration of both intracellular ER and rapid signaling (as has been described in [1–3] and herein). In this review, the model system that we utilize to investigate putative mechanisms of E2 will be described. This will be followed by a discussion of the evidence that E2 has rapid/membrane-mediated effects, actions involving cognate ERs, and a possible integration of these classical and non-classical effects, for behavioral processes mediated by the hippocampus.
IN VIVO MODEL UTILIZED TO INVESTIGATE THE MECHANISMS OF E2 FOR ITS FUNCTIONAL EFFECTS
In our laboratory, the mechanism of steroid hormones, such as E2, in brain targets, such as the hippocampus, are investigated using an animal model. E2 has concentration- and regimen-specific functional effects on processes mediated by the hippocampus (e.g. cognitive and affective behavior) [4]. As such, the primary approach that we have utilized to investigate E2’s actions is to remove the main endogenous sources of E2, the ovaries, by ovariectomy (OVX), then E2 can be administered in a dosage- and timeframe-specific manner. To be able to elucidate the site-specific mechanisms of E2, E2 and/or drugs that targets it putative mechanisms, are typically administered directly to the dorsal hippocampus. Behavioral endpoints that are mediated by the hippocampus, such as performance in cognitive tasks (e.g. inhibitory avoidance task) and/or behavior in anxiety tasks (e.g. elevated plus maze) are then assessed. Additional details of this experimental approach can be found in recent reviews [4–6]. Using this paradigm, we have demonstrated that E2, within a physiologically-relevant regimen, produces cognitive-enhancing and anti-anxiety effects through its actions in the hippocampus. Briefly, rats that are naturally-receptive (proestrus) have improved performance in the inhibitory avoidance and elevated plus maze tasks, compared to rats that are in diestrus and have low E2 levels [4,6–7. OVX attenuates the cognitive-enhancing and-anti-anxiety effects observed during proestrus. E2 administered subcutaneously or to the hippocampus in a regimen that produces proestrus-like E2 levels, reverses the effects of OVX [4,6,8–9]. See Figure 1. Given these clear, replicable effects of E2 in this task as well as other hippocampus-mediated tasks, data in this review will be presented as % of OVX, vehicle-administered control. These studies, which characterized some of E2’s effects in the hippocampus for these behaviors, provided a schema for further investigation of the mechanisms of E2 for these behaviors. As such, the results of experiments using this general approach to elucidate mechanisms of E2 in the hippocampus for its functional effects are discussed in the following sections.
Figure 1. Characterization of E2’s effects on hippocampuse-mediated processes.
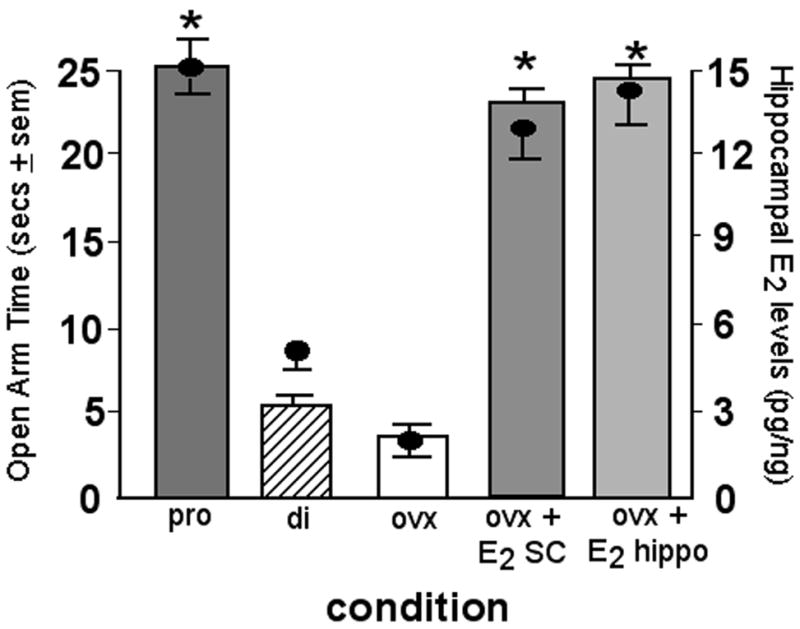
Figure depicts that rats that have proestrus-like, physiological levels of E2 in the hippocampus (proestrous, OVX + E2 subcutaneously (SC) or to the hippocampus (hippo); levels + sem indicated by circles) have improved performance in the elevated plus maze compared to diestrous or OVX rats (behavior indicated by bars; mean secs + sem). * indicates significant difference from diestrous or OVX, vehicle control.
RAPID AND/OR MEMBRANE ACTIONS IN THE HIPPOCAMPUS FOR E2’S FUNCTIONAL EFFECTS
One challenge to elucidating the mechanisms of E2 which may underlie some of its behavioral effects are the diversity of actions of E2. Briefly, E2 can alter neurons, their membranes, the availability of specific membrane receptor proteins, their mitochondrial functions, the production and function of neurotransmitters, and neuromodulators [10–13]. E2 alters astrocyte functions [14] and growth factors [15–17] that influence dendritic branching [18] and synaptogenesis [19]. Some of these processes, and/or others, may account for rapid effects of E2. Because of the plethora of potential actions, the results of several converging approaches that have been utilized to dissociate rapid, membrane actions from those mediated through cognate estrogen receptors are described below.
Some of E2’s effects on behavior that may occur in part independent of direct actions at intracellular ERs, have been referred to as “non-genomic” or non-classical. E2 can have rapid effects within seconds to minutes that are sufficiently fast to preclude actions at intracellular ERs [20]. There are several tools that are typically-used for determining whether E2’s behavioral effects are via rapid, membrane actions that are independent of actions at intracellular ERs. First, a research approach that is utilized to investigate rapid signaling of E2 is a timecourse study. Non-classical effects usually occur in seconds to minutes [21], whereas classical effects are more likely to take hours to days [22]. The minimum latency for transcription, translation, and synthesis of new proteins resulting from steroids’ actions at intracellular steroid receptors is 10–15 minutes [20]. Hence, effects of steroids with a latency of 10 minutes or less are often attributed to non-classical actions. For example, in dissociated hippocampal cells, E2 increases kainate-induced currents in 3 minutes [23]. We have demonstrated that administration of E2 improves affective processes within 10 minutes of administration when administered directly to the hippocampus [24], which supports the idea that some of E2’s effects in the hippocampus for these processes are rapid. This is supported further by a timecourse study that we have done investigating the minimum duration of E2 administration to the hippocampus that is necessary to improve performance in the hippocampus-mediated inhibitory avoidance task. In this study, OVX rats with stereotaxically-implanted cannulae aimed at the dorsal hippocampus were administered E2 via inserts that were filled with crystalline E2 for 5 or 10 minutes following training in this task. Rats were then were tested 24 hours later. We found that exposure of E2 for 10, but not 5, minutes improved performance in this task (see Figure 2). Cell firing in the hippocampus was recorded in rats before and after administration of E2. These studies revealed that enhanced cell firing in the hippocampus were observed at 10 minutes, compared to 5 minutes, following E2 administration, suggesting that these behavioral effects may correspond to increased activity in the hippocampus produced by E2 in this rapid timeframe (see Figure 2). Thus, E2 has rapid actions in the hippocampus.
Figure 2. Rapid functional effects of E2 in the hippocampus.
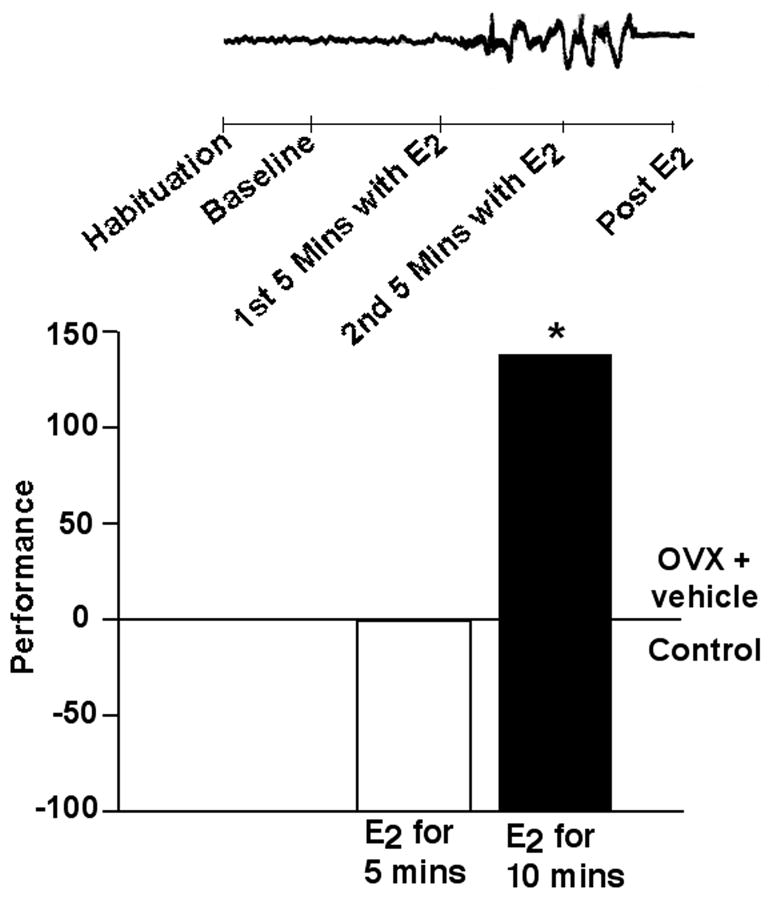
Administration of E2 to the dorsal hippocampus for 10 minutes, but not 5 minutes, following training in the inhibitory avoidance task improves performance. Similar to the pattern of behavioral effects observed with intra-hippocampal administration of E2, cell firing in the hippocampus was increased between 5 and 10 minutes following E2 application. No differences were observed in data collected before E2 administration to the hippocampus (during habituation or baseline), after E2 implants were removed from the hippocampus (data shown in inset above behavioral data), or in an area of the brain that was not administered E2, the cortex (data not shown). Data shown are means expressed as a percent of the control group (age-matched OVX rats administered vehicle to the hippocampus). * indicates significant difference from vehicle control.
A second approach that is often utilized is administration of E2 conjugates that have membrane-relegated effects because E2 is bound to a large macromolecule that cannot readily permeate cell membranes. One example of a typically-utilized E2 conjugate is E2:bovine serum albumin (BSA). It must be noted that there is some concerns that conjugates may be contaminated with free E2 or become unbound and be able to permeate membranes, which would void any interpretation about E2’s actions via cell membranes. However, E2:BSA can be purified by stripping free E2 from the E2 conjugate with charcoal [25]. Moreover, transfection studies have demonstrated the purity of some sources of E2 conjugates [26]. In support, E2:BSA fails to induce an ERE reporter gene in cells transfected with ERs [26]. E2:BSA rapidly alters a signal transduction molecule, mitogen-activated protein kinase (MAPK) [26], which is a likely downstream target of E2’s membrane effects. We have found that administration of free E2 or E2:BSA produces similar effects to enhance inhibitory avoidance performance over that of blank or BSA control implants administered to the hippocampus [9]. See Figure 3. These data suggest that E2 can have membrane actions for its functional effects.
Figure 3. Membrane-mediated functional effects of E2 in the hippocampus.
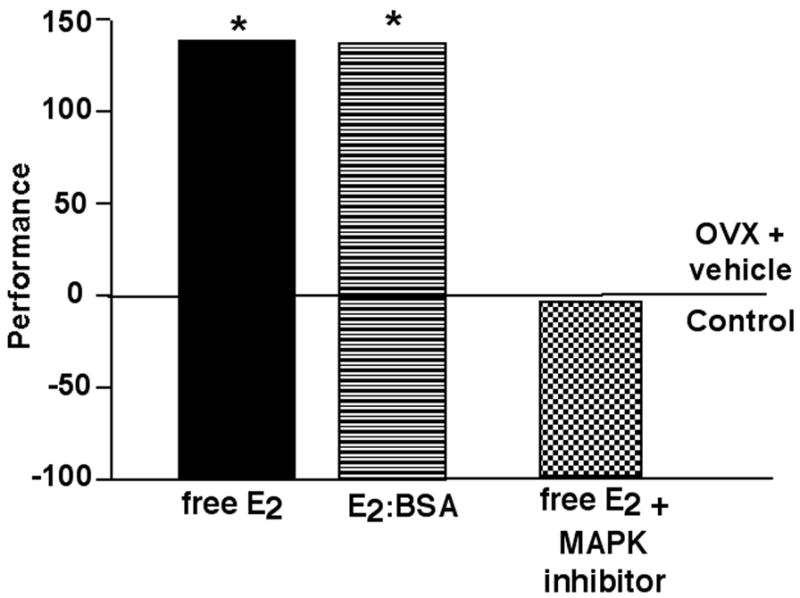
The left side of the figure depicts effects of administration of free E2 or a membrane impermeable E2, E2:BSA, to the dorsal hippocampus following training in the inhibitory avoidance task similarly improving performance. The right side of the figure depicts effects of infusions of a MAPK inhibitor (PD98059) with E2 to the dorsal hippocampus not improving performance in the inhibitory avoidance task, compared to infusions of E2 alone. Data shown are means expressed as a percent of the control group, age-matched OVX rats administered vehicle to the hippocampus. * indicates significant difference from vehicle control.
A third approach that can be taken to investigate the rapid, membrane actions of E2 is to elucidate the downstream mechanisms for these effects. Potential targets for E2’s rapid, non-classical actions include membrane ERs and/or other membrane targets, which likely involve activation of several signal transduction pathways and/or second messengers, such as MAPK, extracellular signal-regulated kinase, phosphatidylinositol-3-kinase, or adenylyl cyclase cascades [27–37]. Indeed, we have found that co-administration of E2 to the hippocampus with a MAPK inhibitor blocked the facilitating actions of E2 for inhibitory avoidance performance (Figure 3). Together, these rapid effects of E2 and the demonstration that inhibiting a signal transduction pathway attenuates these effects suggest that E2 has membrane actions in the hippocampus for some of its functional effects.
A fourth approach that is often taken to investigate whether steroids actions can occur independent of cognate, intracellular steroid receptors is to evaluate the effects of steroids in the absence of receptors. For example, E2 can alter the structure and function of glial cells, even in cells that do not have ERs [398]. To evaluate behavioral effects, the effects of steroids can be ascertained when they are administered directly to brain regions with few cognate receptors. For example, E2 can enhance paced mating behavior when applied to the striatum, an area with few traditional intracellular ERs [39]. Given that the hippocampus has high expression of ERs (as described in detail in the next section), this approach is not tenable in our model. However, using knockout mice that do not express ERs is another approach that can be utilized to determine if ERs are integral for E2’s functional effects. In support, E2 rapidly increases kainate-induced currents in the hippocampus of ER knockout mice [40]. Moreover, some of these studies by Moss and colleagues demonstrated that an ER antagonist, ICI182,780, when applied to the hippocampus did not attenuate effects of E2. However, given the question of the capacity of ICI 182,780 to have antagonistic actions at in the hippocampus at nuclear and/or membrane-associated ERs [36,41], whether some of E2’s rapid/membrane actions may also involve intra-nuclear ERs needs to be addressed.
ESTROGEN-RECEPTOR MEDIATED ACTIONS OF E2 IN THE HIPPOCAMPUS FOR FUNCTIONAL EFFECTS
E2 may have classical actions at intracellular ERs in the hippocampus for its behavioral effects. Intracellular binding sites for E2 were identified nearly 50 years ago [42]. ERs were localized in the rodent brain using autoradiography. Radioactively-labeled E2 injected into female rats is highly concentrated in the hippocampus and other brain areas considered to be important for mediating cognition and/or affect (e.g. medial and cortical amygdala and the lateral septum) [43]. Although these autoradiographic findings were of great importance for localizing hormone target sites, the poor resolution and lack of ability to quantify ERs were serious limitations. Recent studies using in situ hybridization and/or immunocytochemistry with greater resolution indicate that ERs have high expression in the hippocampus [44–45]. Indeed, actions at ERs in the hippocampus may underlie some of E2’s functional effects at this brain target.
ERs function as transcription factors that can be modulated by E2. E2 binds to intracellular ERs in a ligand-dependent manner, resulting in synthesis of proteins that carry out the cell’s functional response [46–47]. To investigate whether E2’s functional effects in the hippocampus required protein synthesis, we co-administered E2 and a protein synthesis inhibitor, cycloheximide, or vehicle, to the hippocampus immediately after training in the inhibitory avoidance task. No differences in performance were observed with co-administration of cycloheximide or vehicle and E2, suggesting that in this paradigm protein synthesis may not be required (Figure 4). However, follow-up studies using ER antagonists, as described next, suggest that some of E2’s functional effects in the hippocampus may require actions at ERs.
Figure 4. Protein synthesis in the hippocampus is not required for E2’s functional effects.
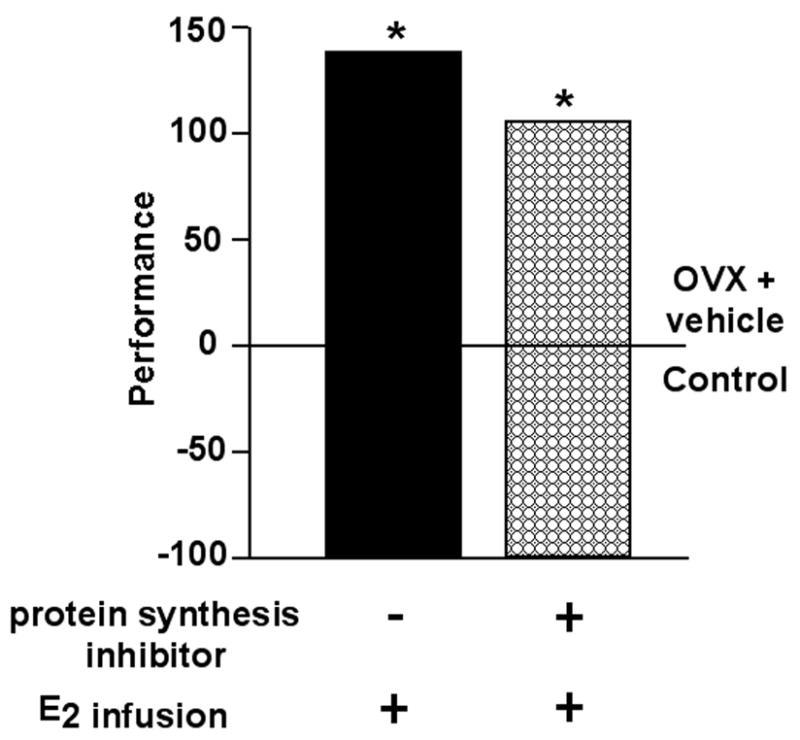
Co-administration of E2 and vehicle, or co-administration of E2 and a protein synthesis inhibitor, to the dorsal hippocampus post-training in the inhibitory avoidance task similarly improves performance. Data shown are means expressed as a percent of the control group, age-matched OVX rats administered vehicle to the hippocampus. * indicates significant difference from vehicle control.
Another way to investigate the ER-dependency of E2’s effects in the hippocampus is by using ER antagonists, such as tamoxifen and ICI182,780. Although there are issues about pharmacological action and tissue- and ER isoform-specificity of tamoxifen and ICI182,780, these compounds are useful in that effects of ER knockdown can be relegated to a specific timeframe, unlike in knockout mouse models that have perturbed ER function throughout development. We have found that administration of E2 SC or to the hippocampus and/or tamoxifen SC or ICI182,780 to the hippocampus similarly improved inhibitory avoidance performance, suggesting that, in this paradigm, activation of ERs was not required [9]. Administration of the ER antagonist, ICI182,780, to the hippocampus, but not the amygdala, attenuated the anti-anxiety and anti-depressive effects of E2 [4]. ICI 182,780 may have effects in the hippocampus to block both nuclear and plasma-membrane associated actions at ERs [36,41. It must be noted that tamoxifen is a less-specific ER ligand that can have both agonistic and antagonistic properties for ER activity, depending on the dosing, tissue targets, as well as other factors. For example, agonistic actions of tamoxifen have been described in bone [48–49], but antagonist effects in breast tissue [50] and brain [51] have also been described. This may explain why systemically administered tamoxifen blocked E2 and SERMs’ effects for affective behavior [52]. Furthermore, tamoxifen has mixed agonist/antagonist actions involving ERα and is described as a pure antagonist of ERβ [53–56]. Despite these caveats of using pharmacological tools to determine the role of ERs for E2’s effects, these data suggest that E2 may have ER-independent and ER-dependent actions in the hippocampus for its effects on cognitive and affective behavior. Given these results, it was important to further investigate the effects of E2 in the hippocampus via intracellular ERs. Indeed, as described above, blocking ERs in the hippocampus via infusions of ICI182,780, attenuates anti-anxiety behavior in the elevated plus maze and other affective behavioral tasks [4]. Although these data suggest that E2’s actions at ERs in the dorsal hippocampus underlie its effects for some hippocampal processes, whether these effects were due to actions at specific ER isoforms was of interest.
ERβ-MEDIATED ACTIONS IN THE HIPPOCAMPUS FOR E2’S FUNCTIONAL EFFECTS
An added level of complexity in the investigation of the molecular mechanisms of E2 for its functional effects in the hippocampus has arisen following the identification of a second isoform of the ER. The original ER identified E2 binding site [42] is now referred to as ERα, after a second form of ER, called ERβ, was identified in rat prostate and uterine tissue [57–58]. ERα and ERβ are homologous in their DNA (97%) and ligand-binding (60%) domains [58], but are located on different chromosomes and with distinct N-terminal regions encoded by these different genes [57–62]. Indeed, there are differences between expression of ERα and ERβ, which suggests that there may be different functional effects of these isoforms.
The expression of ERα and ERβ differs between and within different tissues in the body [44,63]. Some organs (lung, bladder, intestines) and glands (prostate, pituitary) differ in their expression, with ERβ being greater than ERα [60, 64–66]. Notably, within tissues, the distribution of ERα and ERβ can also vary. For example, bone predominantly expresses ERα, except in the marrow which is primarily ERβ[63]. Similarly, in the uterus, ERα is greater but ERβ is expressed in the glandular epithelium [57,67]. A point of particular clinical relevance is that ERα is the target of E2’s trophic effects in mammary and uterine tissue [63]. Thus, we have been investigating the relative role of ERα and ERβ for E2’s functional effects.
In the brain, there are some regional similarities and differences in expression of ERα and ERβ, which support both convergent and divergent actions of E2 and these substrates. ERα and ERβ are both co-expressed in the preoptic area, bed nucleus of the stria terminalis, and medial amygdala [44,68]. However, expression of ERα is greater than ERβ in the ventromedial hypothalamus and pituitary. Moreover, ERβ expression is more predominant than ERα in the cerebral cortex, hippocampus, anterior olfactory nucleus, dorsal raphe, substantia nigra, midbrain ventral tegmental area, and cerebellum [44,45,69]. This distribution of ERα and ERβ, which is overlapping but also shows distinct differences in expression, has substantiated investigation of whether there are functional effects associated with actions at ERα versus ERβ.
To determine whether ERβ in the hippocampus is a target of E2 for its modulation of affective behavior, we compared effects of chronic and acute knock down ERβ. First, cycling or OVX, E2-replaced mice were tested in a variety of tasks to assess affective behavior. Adult, wildtype (WT) and ERβ knockout (βERKO) mice were left intact and tested in proestrus (high physiological E2 levels) or diestrus (low E2 levels) or were OVX and, 44–48 hours before behavioral testing, mice were administered vehicle (vegetable oil) or E2 (0.09 mg/kg, which produces proestrus-like E2 levels; as per [70–72]). Second, the effects of acute knockdown of ERβ were investigated given the potential compensatory mechanisms that can be observed with lifetime knockdown of a gene. As such, adult rats were OVX, implanted with guide cannulae aimed at the left ventricle, primed with 0.09 mg/kg E2 (which produces physiological E2 levels similar to those seen in proestrus), and then tested 44–48 hours later for affective behavior (open field, elevated plus maze, forced swim test). Throughout E2-priming, rats were administered antisense oligodeoxynucleotides (AS-ODNs) targeted toward ERα and/or ERβ or control treatments (saline or scrambled AS-ODN) [73]. To verify that this AS-ODN regimen was effective, expression of ERα and ERβ was determined in the hippocampus and a control site, the ventral medial hypothalamus, by immunocytochemistry of ERα and ERβ. Mice and rats were tested several tasks that measure hippocampus function. In these studies, our hypothesis that E2 has actions in part via ERβ for hippocampal processes was supported. Chronic or acute knockdown of ERβ attenuated the effects of endogenous increases in E2, or administration of E2 to OVX mice or rats, for hippocampally-mediated processes. Mice that were WT, but not βERKO, and had physiological circulating E2 levels had improved affective and cognitive behavior compared to their counterparts that had low E2 levels [4,70–72]. Similar to these effects of chronic knockdown of ERβ in mice over their entire lifetime, administration of ERβ AS-ODNs alone, or co-administered with ERα AS-ODNs, attenuated the anti-anxiety effects of E2-priming to OVX rats. No effects were observed with the administration of a scrambled control or ERα AS-ODN [73]. Indeed, these, and similar AS-ODN manipulations to the hippocampus or striatum, significantly reduced expression of ERs in these regions and others, such as the hypothalamus, as measured by western blotting and/or immunocytochemistry [73–75]. Thus, chronic and acute knock down of ERβ abrogates the effects of E2 for affective behavior.
Because knockout mice have life-long deletion in ERβ, the organizational and activation effects of ERβ cannot be dissociated. As such, it is important that a similar pattern was observed for βERKO mice and rats administered ERβ AS-ODNs for 48 hours of E2-priming. A lack of species differences and similar effects of acute and chronic knockdown of ERβ suggest that this mechanism is physiologically-relevant. However, another way to address some limitations from studies using ERβ knockdown is to investigate effects with administration of ligands that activate ERβ.
Another important consideration to make regarding the efficacy of E2 for modulating hippocampus processes through ERβ is to be able to produce or enhance these effects, rather than simply block them pharmacologically or with transgenic mice as was demonstrated in the previous series of experiments. Behavioral effects of SERMs that have greater affinity for ERβ support the notion that ERβ may be important for E2’s anti-anxiety-like effects. Lifelong administration of phytoestrogens with a greater affinity for ERβ than ERα, such as genistein and daidzein, in the diet of male and female rats increases anti-anxiety behavior in the elevated plus maze [76]. We have found that administration of SERMs SC or to the hippocampus, but not another ERβ-rich brain site, the ventral tegmental area (VTA), of OVX rats improves affective behavior across a variety of tasks, compared to vehicle administration [24,52,77]. Together, these data are further evidence that ERβ is an important receptor target in the hippocampus for specific functional effects involving hippocampal processes.
Although these approaches may support converging evidence for the role of ERβ for E2’s effects in the hippocampus, there are some criticisms with each of these approaches that need to be addressed. For instance, in investigations using ERβ knockout mice or lifelong administration of dietary SERMs, the potential nonspecific and/or compensatory effects (e.g. overexpression of a related gene) due to lifelong deletion or activation of ERβ must be taken into consideration. Indeed, some of the caveats that need to be considered when interpreting data using knockout models are possible pleiotropic effects, developmental abnormalities, and/or production of truncated proteins that have uncertain functional activity. As well, whether functional effects of ERβ are organizational or activational in nature cannot be addressed with these studies given that manipulations occur throughout development and adulthood. As such, the specificity of ERβ as a target for E2’s effects was investigated. To this end, comparisons were made between wildtype and βERKO mice administered vehicle, E2 (0.09 mg/kg), or an ERβ-selective SERM, diarylpropionitrile (DPN; (0.09 mg/kg) for affective and cognitive behavior [70–72]. The results of the present study supported the a priori hypothesis that ERβ in the hippocampus is required for E2’s and ERβ-selective SERMs’ modulation of affective behavior. E2 or DPN administered subcutaneously to OVX WT mice produced similar effects to improve affective behavior in the elevated plus maze. This effect was not observed among mice with chronic knockdown of ERβ. Thus, ERβ is a likely target in the hippocampus for some of E2’s functional effects.
Another consideration to make regarding the functional effects of E2 is the specificity of these effects at ERβ to females. The testosterone metabolite, 3α-androstanediol, may have actions via ERβ [78]. Indeed, we have demonstrated that the performance enhancing effects of 3α-diol are attenuated with co-administration of AS-ODNs that are targeted against ERβ, but not ERα, when administered to the hippocampus [74]. Furthermore, we have found that males can be responsive to ERβ ligands. We compared the effects of administration of E2, ERα-(PPT, 17α-E2), and ERβ-(DPN, coumestrol) SERMs (0.09 mg/kg; SC) to intact, aged (18–22 months old) male congenic mice on a C57BL6J background to vehicle for performance in a variety of tasks mediated by the hippocampus. Similar to what we have observed in OVX rodents, administration of E2, DPN, and coumestrol improved performance in tasks mediated by the hippocampus compared to vehicle administration (Figure 5). As well, differences were not observed in an activity monitor, suggesting that behavioral effects observed were not due to non-specific effects on motor behavior. No differences were observed between males administered vehicle and those administered ERα-SERMs (data not shown). Given the relevance of the mechanisms of androgens as they pertain to prostate cancer, further investigation of these effects at ERβ in males are underway. Together, these data provide further support of the role of ERβ in the hippocampus for some of E2’s functional effects. Another factor to consider in relation to the findings from the present series of experiments is the role of ERα and how this may be integrated with actions of ERβ.
Figure 5. ERβ in the hippocampus is important for E2’s functional effects.
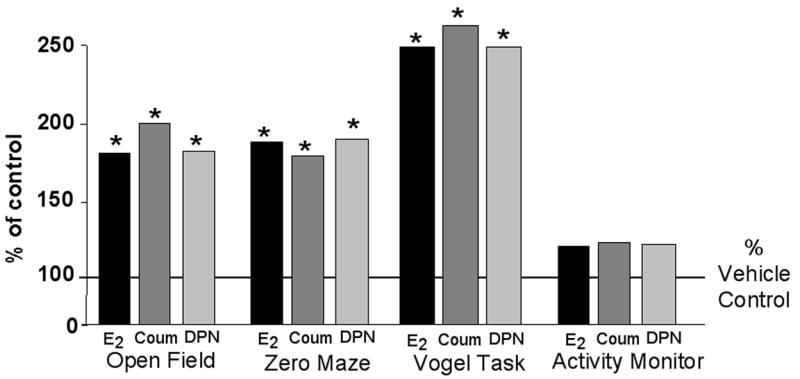
Administration of E2 and ERβ-SERMs (DPN, coumestrol-COUM) improve performance of intact, aged male mice, but do not alter general motor behavior in an activity monitor. Data shown are means expressed as a percent of the control group, age-matched mice administered vehicle. * indicates significant difference from vehicle control.
THE ROLE OF ERα (AND ERβ) FOR FUNCTIONAL EFFECTS OF ESTOGENS
Differential localization of ERα and ERβ support the notion that E2 may have distinct functional effects at these ER isoforms. The studies discussed herein have suggested that an important functional role for ERβ is its effects in the hippocampus. On the other hand, ERα may regulate E2–facilitated sexual behavior in rodents through actions in the hypothalamus. ERα knockout mice have reproductive dysfunction [79–80] and knocking down ERα in the hypothalamus of female mice with an adeno-associated virus vector expressing a small hairpin RNA targeting ERα [81]. In the studies that are described in the previous sections, we utilized sexual receptivity as a control measure. These studies validated our findings with ERβ manipulations, showed that ERα manipulations were effective, and that differences observed were differences in functional response, not non-specific effects of the ER isoform manipulations. We found that SC administration of E2 or ERα-selective SERMs, enhance sexual behavior [52,82]. Furthermore, knockdown of ERα in the hypothalamus attenuates acute effects of E2 to facilitate sexual behavior among OVX rats [73]. Although these data imply distinct functional effects of these receptors, the interaction of these ER subtypes for function is of interest. For example, βERKO mice do not show the same levels of sexual dysfunction as ERα mice demonstrate, and, furthermore, have slightly enhanced sexual receptivity than do their WT counterparts [83]. ERβ-selective phytoestrogens (e.g. genistein, daidzien, coumestrol) attenuate sexual receptivity of female rodents when administered neonatally [84–85]. Other studies in support of the idea that some of these functional effects of E2 may involve integrated actions of ERα and ERβ are those in which both receptors are expressed.
ERα and/or ERβ may be involved in the proliferative effects of E2 in the hippocampus. Proliferating cells can co-express ERα or ERβ and neuronal markers, such as doublecortin, in the hippocampus [86–88]. Administration of E2 or the ERβ-SERM, DPN, increased cell proliferation in the hippocampus of adult female rats as did the ERα-SERM, PPT, at the moderate dosing utilized [88]. Interestingly, cell proliferation was reduced with co-administration of DPN and PPT [88]. Furthermore, ERα in the hippocampus has been suggested to influence neuronal morphology by stimulating dendritic branching [89], whereas spatial, hippocampus-dependent learning has been shown to be mediated by both ERα [90–91] and ERβ [82,92–93].
Evidence for co-localization of ERα and ERα within the same brain region and in the same cells leaves the possibility that some of the differential effects of activating these mechanisms may be due to their role as ERα or ERβ homodimers or ERα/β heterodimers. [94]. Whether ERs act as homodimers versus heterodimers for these effects requires further investigation. Furthermore, ERβ has demonstrated effects to enhance or inhibit ERα activity in in vitro and in vivo systems [95–99]. However, there is evidence that ERβ expression is regulated by activation of ERα [100]. Notably, both ERα and ERβ regulate unique downstream genes and signaling pathways [101]. Thus, these intricacies of some of the factors related to E2 signaling though ERs and other substrates will be investigated in the near future.
Taken together the results of these experiments support non-classical and classical actions of E2 in the hippocampus. Indeed, these results leave open the possibility that E2’s modulation of hippocampally-mediated processes may involve an integration of both membrane and intracellular ER actions. A discussion of the data in support of this notion is as follows.
POTENTIAL INTEGRATION OF RAPID AND ER-MEDIATED SIGNALING IN THE HIPPOCAMPUS FOR E2’s FUNCTIONAL EFFECTS
We have used several experimental approaches (i.e. pharmacological blockers/ligands, transgenic mice, expression studies) to demonstrate that E2’s functional effects require ERβ in the hippocampus for affective behavior. We also have evidence that some of E2’s effects in the hippocampus may be rapid and/or membrane-mediated. This has spurred the question of whether some of these hippocampal processes may be modulated by an integration of E2’s effects at both non-classical and classical substrates. Indeed, it may be that membrane-mediated effects of E2 potentiate classical actions at ERs (See Figure 6). This idea has been thoroughly discussed in a recent review (see [3]). In brief, in vivo and in vitro work demonstrates that E2 may act at membrane ERs that potentiate its effects through nuclear ERs and require activation of protein kinase A or C for its effects [1–3]. Administration of E2:BSA followed by a pulse of free E2 results in a significant increase in luciferase activity in vitro and lordosis when applied to the hypothalamus, compared to administration of free E2 alone [1–2]. A question is whether E2’s effects for hippocampal processes, such as affective and cognitive behavior, may be via an integration of non-classical actions of E2 that prime classical actions.
Figure 6. Possible mechanisms of E2’s action in the hippocampus for affective behavior.
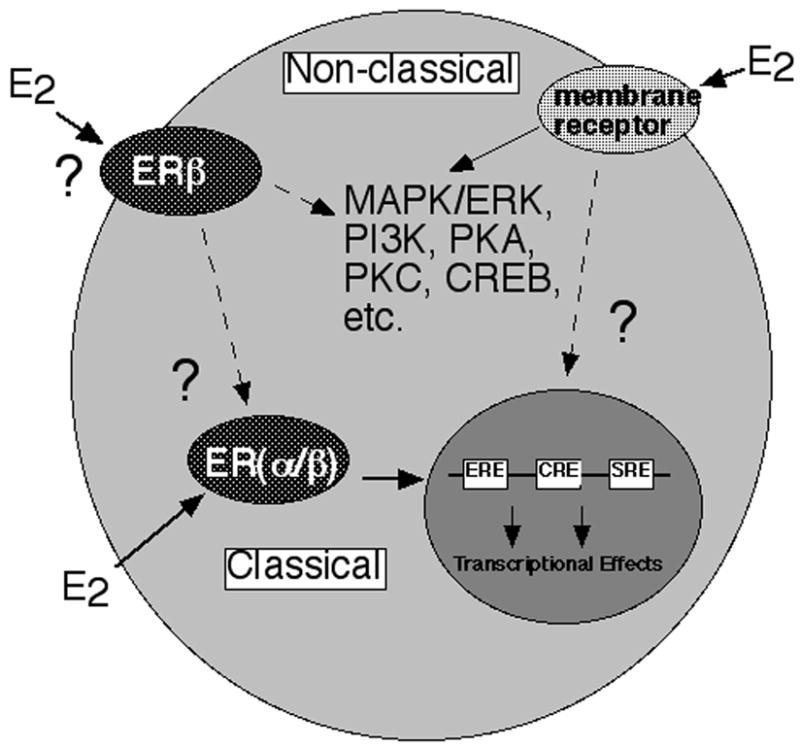
Data have supported that E2 may have direct genomic (or “classical”) effects involving ERβ. E2 may also have indirect genomic effects where binding of ERβ may lead to activation of signal transduction pathways. E2 may bind membrane receptors and activate these signaling pathways. Alternatively, there may be an integration of E2’s actions at membrane and intracellular targets for functional effects of E2 in the hippocampus.
Differences that we have found for the mechanisms of E2 in the hippocampus and amygdala provide some indirect evidence that E2 may have both membrane and intracellular actions for cognitive or affective behavior. Although this review has focused on the hippocampus as a target for E2’s effects, other sites, such as the amygdala, are targets of E2 and involved in cognitive and affective behavior, and deserve mention here. For example, administration of E2 to the hippocampus or amygdala produce similar improvements in affective behavior as does systemic administration of E2 [4,24]. Although these data support the notion that the amygdala is one target of E2, administration of an ER antagonist to the hippocampus, but not the amygdala, blocked the effects of endogenous E2 for affective behavior [4]. As such, a possibility to investigate more in the future is the site-specific mechanisms of E2 in these regions for affective processes. Thus, it may be that E2’s functional effects involve its binding at membrane targets and subsequent activation of downstream signaling molecules in the amygdala that may potentiate actions of E2 at ERs (and/or membrane targets) in the hippocampus.
Studies using SERMs provide additional support that E2’s functional effects due to an integration of rapid, membrane and intracellular ER actions for hippocampal processes. As was described in detail elsewhere [24], administration of E2 as well as ERβ ligands (e.g. DPN, coumestrol) improve affective behavior within 10 minutes when administered directly to the hippocampus. This short-time frame suggests that these effects may involve a membrane-mediated mechanism and/or leave open the possibility that E2’s effects occur with an integration of both rapid, membrane and intracellular ERβ actions in the hippocampus. Another important aspect of this system to consider is that ERs can translocate to the membrane for a relatively brief time and, thus, this may confer some of the rapidity of E2’s actions [102]. This has been shown to be robust for actions of ERβ [103–104]. Indeed, future studies will further investigate how membrane actions of E2 may potentiate intracellular ER activation for E2’s functional effects in the hippocampus.
CONCLUSIONS
There are a number of pathways by which E2 may have rapid signaling integrated with classic actions involving cognate ERs. The data reviewed suggest that ERβ and/or actions at membrane substrates are critical for E2’s functional effects in the hippocampus. Experiments are underway to further establish the role of ERβ and rapid non-ERβ signaling as well as their integrative actions, and downstream targets of these pathways, for hippocampal processes. Experiments, such as these, are timely given that there is a growing disproportion in prevalence of disorders related to hippocampal integrity (e.g. anxiety, mood, cognitive aging, Alzheimer’s Disease) among men and women. Although there is some evidence that E2-based therapies may provide some relief to women, not all studies find this and serious side effects related to cell proliferation limit their usage in some women. Indeed, development of more efficacious therapies with fewer side effects is of interest and will be informed by basic studies investigating the mechanisms of E2’s effects for hippocampal processes and cell proliferation in the body and brain.
Acknowledgments
Research was supported in part by grants from the National Science Foundation (IBN98-96263; IBN03-16083; DBI 00-97342), National Institute of Mental Health (MH0676980), Whitehall Foundation (096-010), Ronald McNair Research Program to support minority undergraduates, and a predoctoral grant from the USAMRMC Dept. of Defense Breast Cancer Research Program (BC051001). Technical assistance provided by Dr. Madeline Rhodes is appreciated. The authors have no conflicts of interest to disclose.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Vasudevan N, Kow LM, Pfaff DW. Early membrane estrogenic effects required for full expression of slower genomic actions in a nerve cell line. PNAS. 2001;98:12267–71. doi: 10.1073/pnas.221449798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Vasudevan N, Kow LM, Pfaff D. Integration of steroid hormone initiated membrane action to genomic function in the brain. Steroids. 2005;70:388–96. doi: 10.1016/j.steroids.2005.02.007. [DOI] [PubMed] [Google Scholar]
- 3.Vasudevan N, Pfaff DW. Membrane-initiated actions of estrogens in neuroendocrinology: emerging principles. Endocr Rev. 2007;28:1–1. doi: 10.1210/er.2005-0021. [DOI] [PubMed] [Google Scholar]
- 4.Walf AA, Frye CA. A review and update of mechanisms of estrogen in the hippocampus and amygdala for anxiety and depression behavior. Neuropsychopharmacology. 2006;31:1097–111. doi: 10.1038/sj.npp.1301067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Walf AA, Frye CA. The use of the elevated plus maze as an assay of anxiety-related behavior in rodents. Nat Protoc. 2007;2:322–8. doi: 10.1038/nprot.2007.44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Rhodes ME, Frye CA. Estrogen has mnemonic-enhancing effects in the inhibitory avoidance task. Pharmacol Biochem Behav. 2006;78:551–8. doi: 10.1016/j.pbb.2004.03.025. [DOI] [PubMed] [Google Scholar]
- 7.Frye CA, Petralia SM, Rhodes ME. Estrous cycle and sex differences in performance on anxiety tasks coincide with increases in hippocampal progesterone and 3α,5α-THP. Pharmacol Biochem Behav. 2000;67:587–596. doi: 10.1016/s0091-3057(00)00392-0. [DOI] [PubMed] [Google Scholar]
- 8.Walf AA, Frye CA. Antianxiety and antidepressive behaviour produced by physiological oestradiol regimen may be modulated by hypothalamic-pituitary-adrenal axis activity. Neuropsychopharmacology. 2005;30:1288–301. doi: 10.1038/sj.npp.1300708. [DOI] [PubMed] [Google Scholar]
- 9.Frye CA, Rhodes ME. Enhancing effects of estrogen on inhibitory avoidance performance may be in part independent of intracellular estrogen receptors in the hippocampus. Brain Res. 2002;956:285–93. doi: 10.1016/s0006-8993(02)03559-x. [DOI] [PubMed] [Google Scholar]
- 10.Bettini E, Maggi A. Estrogen induction of cytochrome c oxidase subunit III in rat hippocampus. J Neurochem. 1992;58:1923–9. doi: 10.1111/j.1471-4159.1992.tb10070.x. [DOI] [PubMed] [Google Scholar]
- 11.Gibbs RB, Gabor R, Cox T, Johnson DA. Effects of raloxifene and estradiol on hippocampal acetylcholine release and spatial learning in the rat. Psychoneuroendocrinology. 2004;29:741–8. doi: 10.1016/S0306-4530(03)00118-5. [DOI] [PubMed] [Google Scholar]
- 12.McCarthy MM, Felzenberg E, Robbins A, Pfaff DW, Schwartz-Giblin S. Infusions of diazepam and allopregnanolone into the midbrain central gray facilitate open-field behavior and sexual receptivity in female rats. Horm Behav. 1995;29:279–95. doi: 10.1006/hbeh.1995.1020. [DOI] [PubMed] [Google Scholar]
- 13.Toran-Allerand CD. Novel sites and mechanisms of oestrogen action in the brain. Novartis Found Symp. 2000;230:56–69. doi: 10.1002/0470870818.ch6. [DOI] [PubMed] [Google Scholar]
- 14.Mong JA, Blutstein T. Estradiol modulation of astrocytic form and function: implications for hormonal control of synaptic communication. Neuroscience. 2006;138:967–75. doi: 10.1016/j.neuroscience.2005.10.017. [DOI] [PubMed] [Google Scholar]
- 15.Duenas M, Luquin S, Chowen JA, Torres-Aleman I, Naftolin F, Garcia-Segura LM. Gonadal hormone regulation of insulin-like growth factor-I-like immunoreactivity in hypothalamic astroglia of developing and adult rats. Neuroendocrinology. 1994;59:528–38. doi: 10.1159/000126702. [DOI] [PubMed] [Google Scholar]
- 16.Gibbs RB. Levels of trkA and BDNF mRNA, but not NGF mRNA, fluctuate across the estrous cycle and increase in response to acute hormone replacement. Brain Res. 1998;787:259–68. doi: 10.1016/s0006-8993(97)01511-4. [DOI] [PubMed] [Google Scholar]
- 17.Singh M, Meyer EM, Simpkins JW. The effect of ovariectomy and estradiol replacement on brain-derived neurotrophic factor messenger ribonucleic acid expression in cortical and hippocampal brain regions of female Sprague-Dawley rats. Endocrinology. 1995;136:2320–4. doi: 10.1210/endo.136.5.7720680. [DOI] [PubMed] [Google Scholar]
- 18.Shughrue PJ, Dorsa DM. Estrogen modulates the growth-associated protein GAP-43 (Neuromodulin) mRNA in the rat preoptic area and basal hypothalamus. Neuroendocrinology. 1993;57:43. doi: 10.1159/000126390. [DOI] [PubMed] [Google Scholar]
- 19.Ferreira A, Caceres A. Estrogen-enhanced neurite growth: evidence for a selective induction of Tau and stable microtubules. J Neurosci. 1991;11:392–400. doi: 10.1523/JNEUROSCI.11-02-00392.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Pfaff DW, McEwen BS. Actions of estrogens and progestins on nerve cells. Science. 1983;219:808–14. doi: 10.1126/science.6297008. [DOI] [PubMed] [Google Scholar]
- 21.Frye CA, DeBold JF. Muscimol facilitates sexual receptivity in hamsters when infused into the ventral tegmentum. Pharmacol Biochem Behav. 1992;42:879–87. doi: 10.1016/0091-3057(92)90044-g. [DOI] [PubMed] [Google Scholar]
- 22.McEwen BS. Interactions between hormones and nerve tissue. Sci Am. 1976;235:48–58. doi: 10.1038/scientificamerican0776-48. [DOI] [PubMed] [Google Scholar]
- 23.Gu Q, Moss RL. 17β-estradiol potentiates kainate-induced currents via activation of the cAMP cascade. J Neurosci. 1996;16:3620–9. doi: 10.1523/JNEUROSCI.16-11-03620.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Walf AA, Frye CA. Administration of estrogen receptor b-specific selective estrogen receptor modulators to the hippocampus decrease anxiety and depressive behavior of ovariectomized rats. Pharmacol Biochem Behav. 2007;86:407–14. doi: 10.1016/j.pbb.2006.07.003. [DOI] [PubMed] [Google Scholar]
- 25.Stevis PE, Deecher DC, Suhadolnik L, Mallis LM, Frail DE. Differential effects of estradiol and estradiol-BSA conjugates. Endocrinology. 1999;140:5455–8. doi: 10.1210/endo.140.11.7247. [DOI] [PubMed] [Google Scholar]
- 26.Watters JJ, Campbell JS, Cunningham MJ, Krebs EG, Dorsa DM. Rapid membrane effects of steroids in neuroblastoma cells: effects of estrogen on mitogen activated protein kinase signaling cascade and c-fos immediate early gene transcription. Endocrinol. 1997;138:4030–3. doi: 10.1210/endo.138.9.5489. [DOI] [PubMed] [Google Scholar]
- 27.Beyer C, Ivanova T, Karolczak M, Kuppers E. Cell type-specificity of nonclassical estrogen signaling in the developing midbrain. J Steroid Biochem Mol Biol. 2002;81:319–25. doi: 10.1016/s0960-0760(02)00119-x. [DOI] [PubMed] [Google Scholar]
- 28.Honda K, Sawada H, Kihara T, Urushitani M, Nakamizo T, Akaike A, Shimohama S. Phosphatidylinositol 3-kinase mediates neuroprotection by estrogen in cultured cortical neurons. J Neurosci Res. 2000;60:321–327. doi: 10.1002/(SICI)1097-4547(20000501)60:3<321::AID-JNR6>3.0.CO;2-T. [DOI] [PubMed] [Google Scholar]
- 29.Kelly MJ, Levin ER. Rapid actions of plasma membrane estrogen receptors. Trends Endocrinol Metab. 2001;12:152–6. doi: 10.1016/s1043-2760(01)00377-0. [DOI] [PubMed] [Google Scholar]
- 30.Linford N, Wade C, Dorsa D. The rapid effects of estrogen are implicated in estrogen-mediated neuroprotection. J Neurocytol. 2000;29:367–74. doi: 10.1023/a:1007113323582. [DOI] [PubMed] [Google Scholar]
- 31.Migliaccio A, Di Domenico M, Castoria G, de Falco A, Bontempo P, Nola E, Auricchio F. Tyrosine kinase/p21ras/MAP-kinase pathway activation by estradiol-receptor complex in MCF-7 cells. EMBO J. 1996;15:1292–300. [PMC free article] [PubMed] [Google Scholar]
- 32.Moss RL, Gu Q. Estrogen: mechanisms for a rapid action in CA1 hippocampal neurons. Steroids. 1999;64:14–21. doi: 10.1016/s0039-128x(98)00092-0. [DOI] [PubMed] [Google Scholar]
- 33.Murphy DD, Segal M. Morphological plasticity of dendritic spines in central neurons is mediated by activation of cAMP response element binding protein. Proc Natl Acad Sci U S A. 1997;94:1482–7. doi: 10.1073/pnas.94.4.1482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Nilsen J, Chen S, Brinton RD. Dual action of estrogen on glutamate-induced calcium signaling: mechanisms requiring interaction between estrogen receptors and src/mitogen activated protein kinase pathway. Brain Res. 2002;930:216–34. doi: 10.1016/s0006-8993(02)02254-0. [DOI] [PubMed] [Google Scholar]
- 35.Roepke TA, Malyala A, Bosch MA, Kelly MJ, Rønnekleiv OK. Estrogen regulation of genes important for K+ channel signaling in the arcuate nucleus. Endocrinology. 2007;148:4937–51. doi: 10.1210/en.2007-0605. [DOI] [PubMed] [Google Scholar]
- 36.Wade CB, Robinson S, Shapiro RA, Dorsa DM. Estrogen receptor (ER)α and ERβ exhibit unique pharmacologic properties when coupled to activation of the mitogen-activated protein kinase pathway. Endocrinol. 2001;142:2336–42. doi: 10.1210/endo.142.6.8071. [DOI] [PubMed] [Google Scholar]
- 37.Zhou Y, Watters JJ, Dorsa DM. Estrogen rapidly induces the phosphorylation of the cAMP response element binding protein in rat brain. Endocrinol. 1996;137:2163–6. doi: 10.1210/endo.137.5.8612562. [DOI] [PubMed] [Google Scholar]
- 38.Mhyre AJ, Dorsa DM. Estrogen activates rapid signaling in the brain: role of estrogen receptor alpha and estrogen receptor beta in neurons and glia. Neuroscience. 2006;138:851–8. doi: 10.1016/j.neuroscience.2005.10.019. [DOI] [PubMed] [Google Scholar]
- 39.Xiao L, Becker JB. Hormonal activation of the striatum and the nucleus accumbens modulates paced mating behavior in the female rat. Horm Behav. 1997;32:114–24. doi: 10.1006/hbeh.1997.1412. [DOI] [PubMed] [Google Scholar]
- 40.Gu Q, Korach KS, Moss RL. Rapid action of 17β-estradiol on kainate-induced currents in hippocampal neurons lacking intracellular estrogen receptors. Endocrinol. 1999;140:660–6. doi: 10.1210/endo.140.2.6500. [DOI] [PubMed] [Google Scholar]
- 41.Wong M, Moss RL. Electrophysiological evidence for a rapid membrane action of the gonadal steroid, 17 β-estradiol, on CA1 pyramidal neurons of the rat hippocampus. Brain Res. 1991;543:148–52. doi: 10.1016/0006-8993(91)91057-8. [DOI] [PubMed] [Google Scholar]
- 42.Jensen EV, Jacobson HI. Basic guides to the mechanism of estrogen action. Recent Prog Horm Res. 1962;18:387–414. [Google Scholar]
- 43.Pfaff D, Keiner M. Atlas of estradiol-concentrating cells in the central nervous system of the female rat. J Comp Neurol. 1973;151:121–58. doi: 10.1002/cne.901510204. [DOI] [PubMed] [Google Scholar]
- 44.Shughrue PJ, Lane MV, Merchenthaler I. Comparative distribution of estrogen receptorα and -β mRNA in the rat central nervous system. J Comp Neurol. 1997;388:507–25. doi: 10.1002/(sici)1096-9861(19971201)388:4<507::aid-cne1>3.0.co;2-6. [DOI] [PubMed] [Google Scholar]
- 45.Shughrue PJ, Scrimo PJ, Merchenthaler I. Evidence for the colocalization of estrogen receptor-β mRNA and estrogen receptor-α immunoreactivity in neurons of the rat forebrain. Endocrinology. 1998;139:5267–70. doi: 10.1210/endo.139.12.6525. [DOI] [PubMed] [Google Scholar]
- 46.Falkenstein E, Tillman HC, Christ M, Feuring M, Wehling M. Multiple actions of steriod hormones--a focus on rapid, nongenomic effects. Pharmacol Rev. 2000;52:513–56. [PubMed] [Google Scholar]
- 47.O’Malley BW, Means AR. Female steroid hormones and target cell nuclei. Science. 1974;183:610–20. doi: 10.1126/science.183.4125.610. [DOI] [PubMed] [Google Scholar]
- 48.Moon LY, Wakley GK, Turner RT. Dose-dependent effects of tamoxifen on long bones in growing rats: influence of ovarian status. Endocrinology. 1991;129:1568–74. doi: 10.1210/endo-129-3-1568. [DOI] [PubMed] [Google Scholar]
- 49.Turner RT, Wakley GK, Hannon KS, Bell NH. Tamoxifen prevents the skeletal effects of ovarian hormone deficiency in rats. J Bone Miner Res. 1987;2:449–56. doi: 10.1002/jbmr.5650020513. [DOI] [PubMed] [Google Scholar]
- 50.Jordan VC. Alternate antiestrogens and approaches to the prevention of breast cancer. J Cell Biochem Suppl. 1995;22:51–7. doi: 10.1002/jcb.240590808. [DOI] [PubMed] [Google Scholar]
- 51.Hilton GD, Ndubuizu AN, McCarthy MM. Neuroprotective effects of estradiol in newborn female rat hippocampus. Brain Res Dev Brain Res. 2004;150:191–8. doi: 10.1016/j.devbrainres.2004.03.006. [DOI] [PubMed] [Google Scholar]
- 52.Walf AA, Frye CA. ERβ-Selective estrogen receptor modulators produce antianxiety behavior when administered systemically to ovariectomized rats. Neuropsychopharmacology. 2005;30:1598–609. doi: 10.1038/sj.npp.1300713. [DOI] [PubMed] [Google Scholar]
- 53.Barkhem T, Carlsson B, Nilsson Y, Enmark E, Gustafsson J, Nilsson S. Differential response of estrogen receptor α and estrogen receptor β to partial estrogen agonists/antagonists. Mol Pharmacol. 1998;54:105–12. doi: 10.1124/mol.54.1.105. [DOI] [PubMed] [Google Scholar]
- 54.Buteau-Lozano H, Ancelin M, Lardeux B, Milanini J, Perrot-Applanat M. Transcriptional regulation of vascular endothelial growth factor by estradiol and tamoxifen in breast cancer cells: a complex interplay between estrogen receptors α and β. Cancer Res. 2002;62:497. [PubMed] [Google Scholar]
- 55.McInerney EM, Katzenellenbogen BS. Different regions in activation function-1 of the human estrogen receptor required for antiestrogen- and estradiol-dependent transcription activation. J Biol Chem. 1996;271:24172–8. doi: 10.1074/jbc.271.39.24172. [DOI] [PubMed] [Google Scholar]
- 56.Zou A, Marschke KB, Arnold KE, Berger EM, Fitzgerald P, Mais DE, Allegretto EA. Estrogen receptor β activates the human retinoic acid receptor α-1 promoter in response to tamoxifen and other estrogen receptor antagonists, but not in response to estrogen. Mol Endocrinol. 1999;13:418–30. doi: 10.1210/mend.13.3.0253. [DOI] [PubMed] [Google Scholar]
- 57.Kuiper GG, Enmark E, Pelto-Huikko M, Nilsson S, Gustafsson JA. Cloning of a novel receptor expressed in rat prostate and ovary. Proc Natl Acad Sci U S A. 1996;93:5925–30. doi: 10.1073/pnas.93.12.5925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Tremblay GB, Tremblay A, Copeland NG, Gilbert DJ, Jenkins NA, Labrie F, Giguere V. Cloning, chromosomal localization, and functional analysis of the murine estrogen receptor β. Mol Endocrinol. 1997;11:353–65. doi: 10.1210/mend.11.3.9902. [DOI] [PubMed] [Google Scholar]
- 59.Green S, Walter P, Kumar V, Krust A, Bornert JM, Argos P, Chambon P. Human oestrogen receptor cDNA: sequence, expression and homology to v-erb-A. Nature. 1986;320:134–9. doi: 10.1038/320134a0. [DOI] [PubMed] [Google Scholar]
- 60.Kuiper GG, Carlsson B, Grandien K, Enmark E, Haggblad J, Nilsson S, Gustafsson JA. Comparison of the ligand binding specificity and transcript tissue distribution of estrogen receptors α and β. Endocrinology. 1997;138:863–70. doi: 10.1210/endo.138.3.4979. [DOI] [PubMed] [Google Scholar]
- 61.Mitchner NA, Garlick C, Ben-Jonathan N. Cellular distribution and gene regulation of estrogen receptors α and β in the rat pituitary gland. Endocrinology. 1998;139:3976–83. doi: 10.1210/endo.139.9.6181. [DOI] [PubMed] [Google Scholar]
- 62.Paech K, Webb P, Kuiper GG, Nilsson S, Gustafsson J, Kushner PJ, Scanlan TS. Differential ligand activation of estrogen receptors ERα and ERβ at AP1 sites. Science. 1997;277:1508–10. doi: 10.1126/science.277.5331.1508. [DOI] [PubMed] [Google Scholar]
- 63.Gustafsson JA. What pharmacologists can learn from recent advances in estrogen signalling. Trends Pharmacol Sci. 2003;24:479–85. doi: 10.1016/S0165-6147(03)00229-3. [DOI] [PubMed] [Google Scholar]
- 64.Matthews J, Gustafsson JA. Estrogen signaling: a subtle balance between ER α and ER β. Mol Interv. 2003;3:281–92. doi: 10.1124/mi.3.5.281. [DOI] [PubMed] [Google Scholar]
- 65.Nishihara E, Nagayama Y, Inoue S, Hiroi H, Muramatsu M, Yamashita S, Koji T. Ontogenetic changes in the expression of estrogen receptor α and β in rat pituitary gland detected by immunohistochemistry. Endocrinology. 2000;141:615–20. doi: 10.1210/endo.141.2.7330. [DOI] [PubMed] [Google Scholar]
- 66.Valimaa H, Savolainen S, Soukka T, Silvoniemi P, Makela S, Kujari H, Gustafsson JA, Laine M. Estrogen receptor-β is the predominant estrogen receptor subtype in human oral epithelium and salivary glands. J Endocrinol. 2004;180:55–62. doi: 10.1677/joe.0.1800055. [DOI] [PubMed] [Google Scholar]
- 67.Hiroi H, Inoue S, Watanabe T, Goto W, Orimo A, Momoeda M, Tsutsumi O, Taketani Y, Muramatsu M. Differential immunolocalization of estrogen receptor α and β in rat ovary and uterus. J Mol Endocrinol. 1999;22:37–44. doi: 10.1677/jme.0.0220037. [DOI] [PubMed] [Google Scholar]
- 68.Greco B, Blasberg ME, Kosinski EC, Blaustein JD. Response of ERα-IR and ERβ-IR cells in the forebrain of female rats to mating stimuli. Horm Behav. 2003;43:444–53. doi: 10.1016/s0018-506x(03)00028-x. [DOI] [PubMed] [Google Scholar]
- 69.Creutz LM, Kritzer MF. Mesostriatal and mesolimbic projections of midbrain neurons immunoreactive for estrogen receptor b or androgen receptors in rats. J Comp Neurol. 2004;476:348–362. doi: 10.1002/cne.20229. [DOI] [PubMed] [Google Scholar]
- 70.Goldberg TN, Koonce C, Walf A, Frye C. Estrogen receptor beta is involved in estradiol’s anti-anxiety and anti-depressant-like effects in adult female mice. Society for Neuroscience Abstract. 2007 [Google Scholar]
- 71.Ryan A, Koonce C, Walf AA, Frye CA. Estrogen receptor beta may be required for estradiol’s effects to enhance cognitive performance of female mice. Society for Neuroscience Abstrac. 2007 [Google Scholar]
- 72.Walf AA, Koonce C, Frye CA. Adult female wildtype, but not estrogen receptor β knockout, mice have decreased depression-like behavior during proestrus and following administration of estradiol or diarylpropionitrile. J Psychopharmacology. 2007 doi: 10.1177/0269881108089598. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Walf AA, Ciriza I, Garcia-Segura LM, Frye CA. Antisense oligodeoxynucleotides for estrogen receptor-b and a attenuate estradiol’s modulation of affective and sexual behavior, respectively. Neuropsychopharmacology. 2007 doi: 10.1038/sj.npp.1301416. in press. [DOI] [PubMed] [Google Scholar]
- 74.Edinger KL, Frye CA. Androgens’ effects to enhance learning may be mediated in part through actions at estrogen receptor-b in the hippocampus. Neurobiol Learn Mem. 2007;87:78–85. doi: 10.1016/j.nlm.2006.07.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Walf AA, Rhodes ME, Meade JR, Harney JP, Frye CA. Oestradiol-induced conditioned place preference requires actions at estrogen receptors in the nucleus accumbens. Neuropsychopharmacology. 2007;32:522–30. doi: 10.1038/sj.npp.1301124. [DOI] [PubMed] [Google Scholar]
- 76.Lephart ED, West TW, Weber KS, Rhees RW, Setchell KD, Adlercreutz H, et al. Neuro-behavioral effects of dietary soy phytoestrogens. Neurotoxicol Teratol. 2002;24:5–16. doi: 10.1016/s0892-0362(01)00197-0. [DOI] [PubMed] [Google Scholar]
- 77.Walf AA, Rhodes ME, Frye CA. Anti-depressant effects of ERβ selective estrogen receptor modulators in the forced swim test. Pharmacol Biochem Behav. 2004;78:523–9. doi: 10.1016/j.pbb.2004.03.023. [DOI] [PubMed] [Google Scholar]
- 78.Handa RJ, Pak TR, Kudwa AE, Lund TD, Hinds L. An alternate pathway for androgen regulation of brain function: Activation of estrogen receptor beta by the metabolite of dihydrotestosterone, 5alpha-androstane-3beta, 17beta-diol. Horm Behav. 2007 doi: 10.1016/j.yhbeh.2007.09.012. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Ogawa S, Eng V, Taylor J, Lubahn DB, Korach KS, Pfaff DW. Roles of estrogen receptor-α gene expression in reproduction-related behaviors in female mice. Endocrinology. 1998;139:5070–81. doi: 10.1210/endo.139.12.6357. [DOI] [PubMed] [Google Scholar]
- 80.Ogawa S, Chan J, Gustafsson JA, Korach KS, Pfaff DW. Estrogen increases locomotor activity in mice through estrogen receptor α: specificity for the type of activity. Endocrinology. 2003;144:230–9. doi: 10.1210/en.2002-220519. [DOI] [PubMed] [Google Scholar]
- 81.Musatov S, Chen W, Pfaff DW, Kaplitt MG, Ogawa S. RNAi-mediated silencing of estrogen receptor {α} in the ventromedial nucleus of hypothalamus abolishes female sexual behaviors. PNAS. 2006;103:10456–60. doi: 10.1073/pnas.0603045103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Rhodes ME, Frye CA. ERb-selective SERMs produce mnemonic-enhancing effects in the inhibitory avoidance and water maze tasks. Neurobiol Learn Mem. 2006;85:183–91. doi: 10.1016/j.nlm.2005.10.003. [DOI] [PubMed] [Google Scholar]
- 83.Ogawa S, Chan J, Chester AE, Gustafsson JA, Korach KS, Pfaff DW. Survival of reproductive behaviors in estrogen receptor β gene-deficient (βERKO) male and female mice. Proc Natl Acad Sci U S A. 1999;96:12887–92. doi: 10.1073/pnas.96.22.12887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Kouki T, Kishitake M, Okamoto M, Oosuka I, Takebe M, Yamanouchi K. Effects of neonatal treatment with phytoestrogens, genistein and daidzein, on sex difference in female rat brain function: estrous cycle and lordosis. Horm Behav. 2003;44:140–5. doi: 10.1016/s0018-506x(03)00122-3. [DOI] [PubMed] [Google Scholar]
- 85.Kouki T, Okamoto M, Wada S, Kishitake M, Yamanouchi K. Suppressive effect of neonatal treatment with a phytoestrogen, coumestrol, on lordosis and estrous cycle in female rats. Brain Res Bull. 2005;64:449–54. doi: 10.1016/j.brainresbull.2004.10.002. [DOI] [PubMed] [Google Scholar]
- 86.Isgor C, Watson SJ. Estrogen receptor α and β mRNA expressions by proliferating and differentiating cells in the adult rat dentate gyrus and subventricular zone. Neuroscience. 2005;134:847–56. doi: 10.1016/j.neuroscience.2005.05.008. [DOI] [PubMed] [Google Scholar]
- 87.Herrick SP, Waters EM, Drake CT, McEwen BS, Milner TA. Extranuclear estrogen receptor β immunoreactivity is on doublecortin-containing cells in the adult and neonatal rat dentate gyrus. Brain Res. 2006;1121:46–58. doi: 10.1016/j.brainres.2006.08.084. [DOI] [PubMed] [Google Scholar]
- 88.Mazzucco CA, Lieblich SE, Bingham BI, Williamson MA, Viau V, Galea LA. Both estrogen receptor α and estrogen receptor β agonists enhance cell proliferation in the dentate gyrus of adult female rats. Neuroscience. 2006;141:793–0. doi: 10.1016/j.neuroscience.2006.05.032. [DOI] [PubMed] [Google Scholar]
- 89.Audesirk T, Cabell L, Kern M, Audesirk G. β-estradiol influences differentiation of hippocampal neurons in vitro through an estrogen receptor-mediated process. Neuroscience. 2003;121:927–34. doi: 10.1016/s0306-4522(03)00294-x. [DOI] [PubMed] [Google Scholar]
- 90.Fugger HN, Cunningham SG, Rissman EF, Foster TC. Sex differences in the activational effect of ERalpha on spatial learning. Horm Behav. 1998;34:163–70. doi: 10.1006/hbeh.1998.1475. [DOI] [PubMed] [Google Scholar]
- 91.Frye CA, Duffy CK, Walf AA. Estrogens and progestins enhance spatial learning of intact and ovariectomized rats in the object placement task. Neurobiol Learn Mem. 2007;88:208–16. doi: 10.1016/j.nlm.2007.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Rissman EF, Heck AL, Leonard JE, Shupnik MA, Gustafsson JA. Disruption of estrogen receptor b gene impairs spatial learning in mice. PNAS. 2002;99:3996–01. doi: 10.1073/pnas.012032699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Walf AA, Rhodes ME, Frye CA. Ovarian steroids enhance object recognition in naturally cycling and ovariectomized, hormone-primed rats. Neurobiol Learn Mem. 2006;86:35–46. doi: 10.1016/j.nlm.2006.01.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.McDonnell DP, Connor CE, Wijayaratne A, Chang CY, Norris JD. Definition of the molecular and cellular mechanisms underlying the tissue-selective agonist/antagonist activities of selective estrogen receptor modulators. Recent Prog Horm Res. 2002;57:295–316. doi: 10.1210/rp.57.1.295. [DOI] [PubMed] [Google Scholar]
- 95.Couse JF, Dixon D, Yates M, Moore AB, Ma L, Maas Korach KS. Estrogen receptor-α knockout mice exhibit resistance to the developmental effects of neonatal diethylstilbestrol exposure on the female reproductive tract. Dev Biol. 2001;238:224–38. doi: 10.1006/dbio.2001.0413. [DOI] [PubMed] [Google Scholar]
- 96.Frasor J, Barnett DH, Danes JM, Hess R, Parlow AF, Katzenellenbogen BS. Response-specific and ligand dose-dependent modulation of estrogen receptor (ER) α activity by ERβ in the uterus. Endocrinology. 2003;144:3159–66. doi: 10.1210/en.2002-0143. [DOI] [PubMed] [Google Scholar]
- 97.Hall JM, McDonnell DP. The estrogen receptor b-isoform (ERb) of the human estrogen receptor modulates ERa transcriptional activity and is a key regulator of the cellular response to estrogens and antiestrogens. Endocrinology. 1999;140:5566–78. doi: 10.1210/endo.140.12.7179. [DOI] [PubMed] [Google Scholar]
- 98.Pettersson K, Delaunay F, Gustafsson JA. Estrogen receptor β acts as a dominant regulator of estrogen signaling. Oncogene. 2000;19:4970–8. doi: 10.1038/sj.onc.1203828. [DOI] [PubMed] [Google Scholar]
- 99.Pettersson K, Gustafsson JA. Role of estrogen receptor β in estrogen action. Annu Rev Physiol. 2001;63:165–92. doi: 10.1146/annurev.physiol.63.1.165. [DOI] [PubMed] [Google Scholar]
- 100.Glaeser M, Niederacher D, Djahansouzi S, Hanstein B, Dittrich R, Beckmann MW, et al. Effects of the antiestrogens tamoxifen and raloxifene on the estrogen receptor transactivation machinery. Anticancer Res. 2006;26:735–44. [PubMed] [Google Scholar]
- 101.Hurst AG, Goad DW, Mohan M, Malayer JR. Independent downstream gene expression profiles in the presence of estrogen receptor α or β. Biol Reprod. 2004;71:1252–61. doi: 10.1095/biolreprod.104.029421. [DOI] [PubMed] [Google Scholar]
- 102.Rønnekleiv OK, Malyala A, Kelly MJ. Membrane-initiated signaling of estrogen in the brain. Semin Reprod Med. 2007;25:165–7. doi: 10.1055/s-2007-973429. [DOI] [PubMed] [Google Scholar]
- 103.Shapiro RA, Sheldahl LC, Dorsa DM. Estrogen induces rapid translocation of ERβ, but not ERα, to the plasma membrane in neurons. Society for Neuroscience Abstract. 2007 doi: 10.1016/j.neuroscience.2008.02.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Sheldahl LC, Marriott LK, Bryant DM, Shapiro RA, Dorsa DM. Neuroprotective effects of estrogen and selective estrogen receptor modulators begin at the plasma membrane. Minerva Endocrinol. 2007;32:87–94. [PubMed] [Google Scholar]


