Abstract
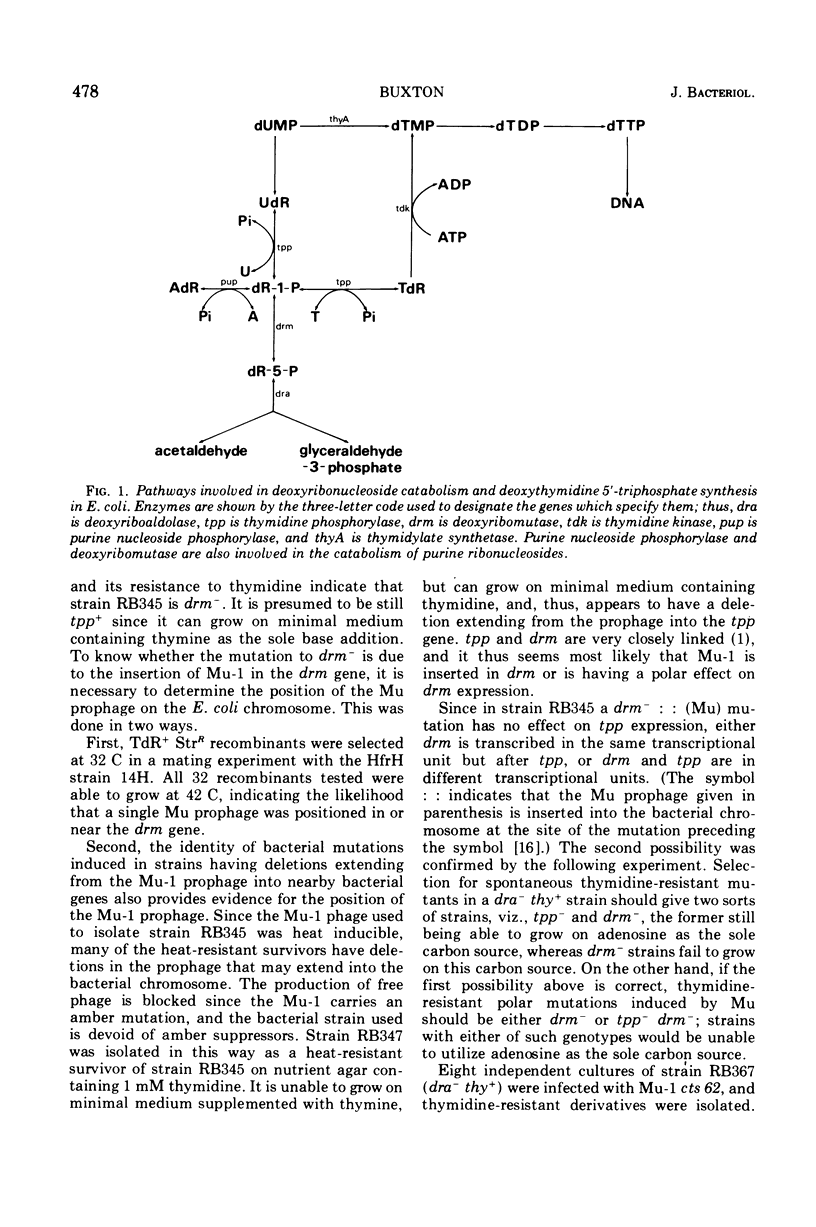
Four genes, dra, tpp, drm, and pup, that specify enzymes involved in the catabolism of nucleosides and deoxynucleosides in Escherichia coli are known to be very closely linked in the order dra-tpp-drm-pup. By infecting cells with the phage Mu-1 and isolating low-thymine-requiring derivatives of a strain lacking thymidylate synthetase and also thymidine-resistant mutants of a dra-strain, it has been possible to select for strains in which Mu-1 is inserted in this gene cluster. Making use of the polar effect of Mu-induced mutations on more distal genes in the same transcriptional unit, evidence is presented that dra and tpp are co-transcribed from a promoter to the left of dra, and drm and pup are co-transcribed from a promotor located between tpp and drm. Residual levels of purine nucleoside phosphorylase in drm- mutants induced by phage Mu seem to indicate that a weak promotor lies between drm and pup. From a strain in which Mu-1 is inserted in drm, a mutant has been isolated that has a deletion extending into tpp. Since this strain lacks thymidylate synthetase, it is unable to grow on minimal medium containing thymine. Mutants isolated from this strain that can grow on minimal medium containing thymine have been shown to have increased levels of the enzyme uridine phosphorylase.
Full text
PDF








Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ahmad S. I., Pritchard R. H. A map of four genes specifying enzymes involved in catabolism of nucleosides and deoxynucleosides in Escherichia coli. Mol Gen Genet. 1969 Aug 15;104(4):351–359. doi: 10.1007/BF00334234. [DOI] [PubMed] [Google Scholar]
- Ahmad S. I., Pritchard R. H. A regulatory mutant affecting the synthesis of enzymes involved in the catabolism of nucleosides in Escherichia coli. Mol Gen Genet. 1971;111(1):77–83. doi: 10.1007/BF00286556. [DOI] [PubMed] [Google Scholar]
- Ahmad S. I., Pritchard R. H. An operator constitutive mutant affecting the synthesis of two enzymes involved in the catabolism of nucleosides in Escherichia coli. Mol Gen Genet. 1973 Aug 28;124(4):321–328. doi: 10.1007/BF00267661. [DOI] [PubMed] [Google Scholar]
- Bachmann B. J. Pedigrees of some mutant strains of Escherichia coli K-12. Bacteriol Rev. 1972 Dec;36(4):525–557. doi: 10.1128/br.36.4.525-557.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barth P. T., Beacham I. R., Ahmad S. I., Pritchard R. H. The inducer of the deoxynucleoside phosphorylases and deoxyriboaldolase in Escherichia coli. Biochim Biophys Acta. 1968 Jul 23;161(2):554–557. doi: 10.1016/0005-2787(68)90132-9. [DOI] [PubMed] [Google Scholar]
- Bauerle R. H., Margolin P. Evidence for two sites for initiation of gene expression in the tryptophan operon of Salmonella typhimurium. J Mol Biol. 1967 Jun 28;26(3):423–436. doi: 10.1016/0022-2836(67)90313-0. [DOI] [PubMed] [Google Scholar]
- Beacham I. R., Beacham K., Zaritsky A., Pritchard R. H. Intracellular thymidine triphosphate concentrations in wild type and in thymine requiring mutants of Escherichia coli 15 and K12. J Mol Biol. 1971 Aug 28;60(1):75–86. doi: 10.1016/0022-2836(71)90448-7. [DOI] [PubMed] [Google Scholar]
- Beacham I. R., Eisenstark A., Barth P. T., Pritchard R. H. Deoxynucleoside-sensitive mutants of Salmonella typhimurium. Mol Gen Genet. 1968;102(2):112–127. doi: 10.1007/BF01789138. [DOI] [PubMed] [Google Scholar]
- Beacham I. R., Pritchard R. H. The role of nucleoside phosphorylases in the degradation of deoxyribonucleosides by thymine-requiring mutants of E. coli. Mol Gen Genet. 1971;110(4):289–298. doi: 10.1007/BF00438271. [DOI] [PubMed] [Google Scholar]
- Bernstein A. The E. coli cell surface: on the genetic organization of the tolPAB cluster. Mol Gen Genet. 1973;123(2):111–121. doi: 10.1007/BF00267328. [DOI] [PubMed] [Google Scholar]
- Buxton R. S., Holland I. B. Genetic studies of tolerance to colicin E2 in Escherichia coli K-12. I. Re-location and dominance relationships of cet mutations. Mol Gen Genet. 1973 Dec 14;127(1):69–88. doi: 10.1007/BF00267784. [DOI] [PubMed] [Google Scholar]
- Daniell E., Roberts R., Abelson J. Mutations in the lactose operon caused by bacteriophage Mu. J Mol Biol. 1972 Aug 14;69(1):1–8. doi: 10.1016/0022-2836(72)90019-8. [DOI] [PubMed] [Google Scholar]
- Gardner R., Kornberg A. Biochemical studies of bacterial sporulation and germination. V. Purine nucleoside phosphorylase of vegetative cells and spores of Bacillus cereus. J Biol Chem. 1967 May 25;242(10):2383–2388. [PubMed] [Google Scholar]
- Guerola N., Ingraham J. L., Cerdá-Olmedo E. Induction of closely linked multiple mutations by nitrosoguanidine. Nat New Biol. 1971 Mar 24;230(12):122–125. doi: 10.1038/newbio230122a0. [DOI] [PubMed] [Google Scholar]
- Howe M. M. Prophage deletion mapping of bacteriophage Mu-1. Virology. 1973 Jul;54(1):93–101. doi: 10.1016/0042-6822(73)90118-9. [DOI] [PubMed] [Google Scholar]
- Jacoby G. A. Control of the argECBH cluster in Escherichia coli. Mol Gen Genet. 1972;117(4):337–348. doi: 10.1007/BF00333027. [DOI] [PubMed] [Google Scholar]
- Jordan E., Saedler H., Starlinger P. O0 and strong-polar mutations in the gal operon are insertions. Mol Gen Genet. 1968;102(4):353–363. doi: 10.1007/BF00433726. [DOI] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Low B. Formation of merodiploids in matings with a class of Rec- recipient strains of Escherichia coli K12. Proc Natl Acad Sci U S A. 1968 May;60(1):160–167. doi: 10.1073/pnas.60.1.160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morse D. E., Yanofsky C. The internal low-efficiency promoter of the tryptophan operon of Escherichia coli. J Mol Biol. 1968 Dec;38(3):447–451. doi: 10.1016/0022-2836(68)90401-4. [DOI] [PubMed] [Google Scholar]
- Munch-Petersen A., Nygaard P., Hammer-Jespersen K., Fiil N. Mutants constitutive for nucleoside-catabolizing enzymes in Escherichia coli K12. Isolation, charactrization and mapping. Eur J Biochem. 1972 May 23;27(2):208–215. doi: 10.1111/j.1432-1033.1972.tb01828.x. [DOI] [PubMed] [Google Scholar]
- Munch-Petersen A. On the catabolism of deoxyribonucleosides in cells and cell extracts of Escherichia coli. Eur J Biochem. 1968 Nov;6(3):432–442. doi: 10.1111/j.1432-1033.1968.tb00465.x. [DOI] [PubMed] [Google Scholar]
- Nomura M., Engbaek F. Expression of ribosomal protein genes as analyzed by bacteriophage Mu-induced mutations. Proc Natl Acad Sci U S A. 1972 Jun;69(6):1526–1530. doi: 10.1073/pnas.69.6.1526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Donovan G. A., Neuhard J. Pyrimidine metabolism in microorganisms. Bacteriol Rev. 1970 Sep;34(3):278–343. doi: 10.1128/br.34.3.278-343.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- RACKER E. Enzymatic synthesis and breakdown of desoxyribose phosphate. J Biol Chem. 1952 May;196(1):347–365. [PubMed] [Google Scholar]
- RAZZELL W. E., KHORANA H. G. Purification and properties of a pyrimidine deoxyriboside phosphorylase from Escherichia coli. Biochim Biophys Acta. 1958 Jun;28(3):562–566. doi: 10.1016/0006-3002(58)90519-5. [DOI] [PubMed] [Google Scholar]
- Robertson B. C., Hoffee P. A. Purification and properties of purine nucleoside phosphorylase from Salmonella typhimurium. J Biol Chem. 1973 Mar 25;248(6):2040–2043. [PubMed] [Google Scholar]
- Robertson B. C., Jargiello P., Blank J., Hoffee P. A. Genetic regulation of ribonucleoside and deoxyribonucleoside catabolism in Salmonella typhimurium. J Bacteriol. 1970 Jun;102(3):628–635. doi: 10.1128/jb.102.3.628-635.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silverman M., Simon M. Genetic analysis of bacteriophage Mu-induced flagellar mutants in Escherichia coli. J Bacteriol. 1973 Oct;116(1):114–122. doi: 10.1128/jb.116.1.114-122.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- TAYLOR A. L. BACTERIOPHAGE-INDUCED MUTATION IN ESCHERICHIA COLI. Proc Natl Acad Sci U S A. 1963 Dec;50:1043–1051. doi: 10.1073/pnas.50.6.1043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Toussaint A. Insertion of phage Mu. 1 within prophage lambda. A new approach for studying the control of the late functions in bacteriophage lambda. Mol Gen Genet. 1969;106(1):89–92. doi: 10.1007/BF00332824. [DOI] [PubMed] [Google Scholar]


