Abstract
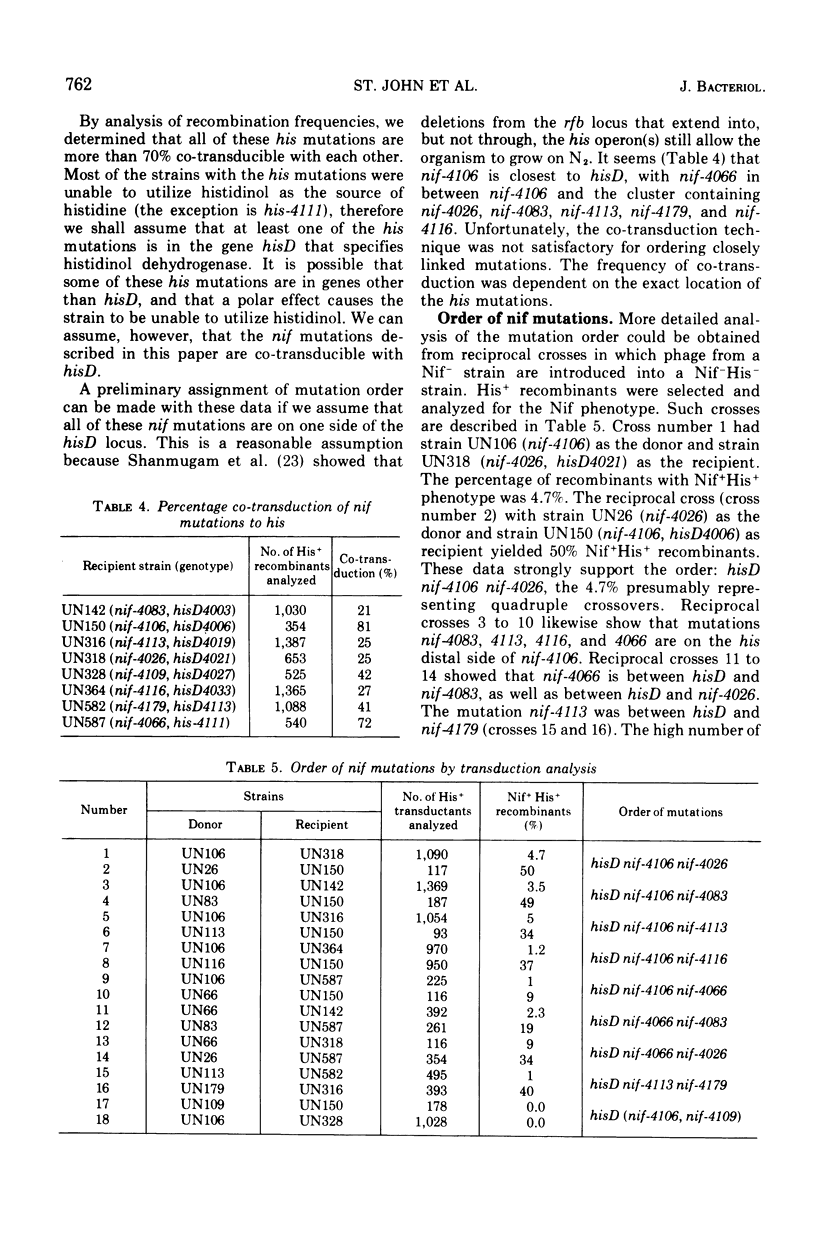
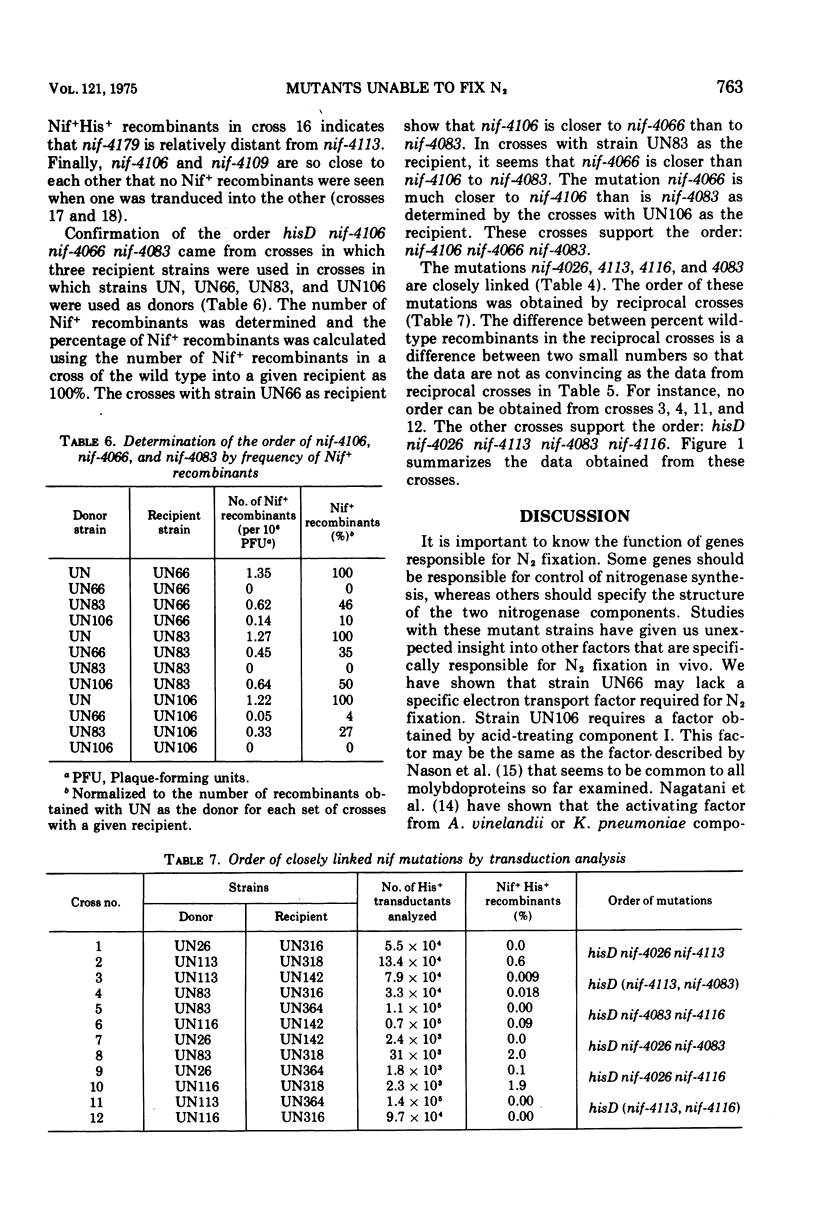
Selected mutant strains of Klebsiella pneumoniae that are unable to fix nitrogen have been characterized according to nitrogenase component activity as well as antigenic cross-reacting material. The lesions in these strains have been mapped by transduction, and the results indicate that there are at least five genes specifically responsible for nitrogen fixation in vivo. Besides genes that specify the structure of the two nitrogenase components, there is a gene for a factor that is required for component I activity and a gene that codes for a factor possibly involved in electron transport to component II. A mutation in another site does not allow the organism to produce either of the nitrogenase components. All of these genes are co-transducible with the gene that specifics the structure of histidinol dehydrogenase.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Brill W. J., Steiner A. L., Shah V. K. Effect of molybdenum starvation and tungsten on the synthesis of nitrogenase components in Klebsiella pneumonia. J Bacteriol. 1974 Jun;118(3):986–989. doi: 10.1128/jb.118.3.986-989.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brill W. J., Westphal J., Stieghorst M., Davis L. C., Shah V. K. Detection of nitrogenase components and other nonheme iron proteins in polyacrylamide gels. Anal Biochem. 1974 Jul;60(1):237–241. doi: 10.1016/0003-2697(74)90149-3. [DOI] [PubMed] [Google Scholar]
- Cannon F. C., Dixon R. A., Postgate J. R., Primrose S. B. Chromosomal integration of Klebsiella nitrogen fixation genes in Escherichia coli. J Gen Microbiol. 1974 Jan;80(1):227–239. doi: 10.1099/00221287-80-1-227. [DOI] [PubMed] [Google Scholar]
- Chang G. W., Straus D., Ames B. N. Enriched selection of dominant mutations: histidine operator mutations. J Bacteriol. 1971 Aug;107(2):578–579. doi: 10.1128/jb.107.2.578-579.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Demerec M., Adelberg E. A., Clark A. J., Hartman P. E. A proposal for a uniform nomenclature in bacterial genetics. Genetics. 1966 Jul;54(1):61–76. doi: 10.1093/genetics/54.1.61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dixon R. A., Postgate J. R. Genetic transfer of nitrogen fixation from Klebsiella pneumoniae to Escherichia coli. Nature. 1972 May 12;237(5350):102–103. doi: 10.1038/237102a0. [DOI] [PubMed] [Google Scholar]
- Dixon R. A., Postgate J. R. Transfer of nitrogen-fixation genes by conjugation in Klebsiella pneumoniae. Nature. 1971 Nov 5;234(5323):47–48. doi: 10.1038/234047a0. [DOI] [PubMed] [Google Scholar]
- Fisher R. J., Brill W. J. Mutants of Azotobacter vinelandii unable to fix nitrogen. Biochim Biophys Acta. 1969 Jun 17;184(1):99–105. doi: 10.1016/0304-4165(69)90103-2. [DOI] [PubMed] [Google Scholar]
- Gordon J. K., Brill W. J. Derepression of nitrogenase synthesis in the presence of excess NH4+. Biochem Biophys Res Commun. 1974 Aug 5;59(3):967–971. doi: 10.1016/s0006-291x(74)80074-4. [DOI] [PubMed] [Google Scholar]
- Gordon J. K., Brill W. J. Mutants that produce nitrogenase in the presence of ammonia. Proc Natl Acad Sci U S A. 1972 Dec;69(12):3501–3503. doi: 10.1073/pnas.69.12.3501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nagatani H. H., Brill W. J. Nitrogenase V. The effect of Mo, W and V on the synthesis of nitrogenase components in Azotobacter vinelandii. Biochim Biophys Acta. 1974 Aug 7;362(1):160–166. doi: 10.1016/0304-4165(74)90037-3. [DOI] [PubMed] [Google Scholar]
- Nagatani H. H., Shah V. K., Brill W. J. Activation of inactive nitrogenase by acid-treated component I. J Bacteriol. 1974 Nov;120(2):697–701. doi: 10.1128/jb.120.2.697-701.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nason A., Lee K. Y., Pan S. S., Ketchum P. A., Lamberti A., DeVries J. Invitro formation of assimilatory reduced nicotinamide adenine dinucleotide phosphate: nitrate reductase from a Neurospora mutant and a component of molybdenum-enzymes. Proc Natl Acad Sci U S A. 1971 Dec;68(12):3242–3246. doi: 10.1073/pnas.68.12.3242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Orme-Johnson W. H., Hamilton W. D., Jones T. L., Tso M. Y., Burris R. H., Shah V. K., Brill W. J. Electron paramagnetic resonance of nitrogenase and nitrogenase components from Clostridium pasteurianum W5 and Azotobacter vinelandii OP. Proc Natl Acad Sci U S A. 1972 Nov;69(11):3142–3145. doi: 10.1073/pnas.69.11.3142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prival M. J., Brenchley J. E., Magasanik B. Glutamine synthetase and the regulation of histidase formation in Klebsiella aerogenes. J Biol Chem. 1973 Jun 25;248(12):4334–4344. [PubMed] [Google Scholar]
- Roth J. R. UGA nonsense mutations in Salmonella typhimurium. J Bacteriol. 1970 May;102(2):467–475. doi: 10.1128/jb.102.2.467-475.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sen M., Sen S. P. Interspecific transformation in Azotobacter. J Gen Microbiol. 1965 Oct;41(1):1–6. doi: 10.1099/00221287-41-1-1. [DOI] [PubMed] [Google Scholar]
- Shah V. K., Davis I. C., Gordon J. K., Orme-Johnson W. H., Brill W. J. Nitrogenase. 3. Nitrogenaseless mutants of Azotobacter vinelandii: activities, cross-reactions and EPR spectra. Biochim Biophys Acta. 1973 Jan 18;292(1):246–255. doi: 10.1016/0005-2728(73)90269-7. [DOI] [PubMed] [Google Scholar]
- Shah V. K., Davis L. C., Stieghorst M., Brill W. J. Mutant of Azotobacter vinelandii that hyperproduces nitrogenase component II. J Bacteriol. 1974 Feb;117(2):917–919. doi: 10.1128/jb.117.2.917-919.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sorger G. J., Trofimenkoff D. Nitrogenaseless mutants of Azotobacter vinelandii. Proc Natl Acad Sci U S A. 1970 Jan;65(1):74–80. doi: 10.1073/pnas.65.1.74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- St John R. T., Shah V. K., Brill W. J. Regulation of nitrogenase synthesis by oxygen in Klebsiella pneumoniae. J Bacteriol. 1974 Jul;119(1):266–269. doi: 10.1128/jb.119.1.266-269.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stodolsky M. Bacteriophage P1 derivatives with bacterial genes: a heterozygote enrichment method for the selection of p1dpro lysogens. Virology. 1973 Jun;53(2):471–475. doi: 10.1016/0042-6822(73)90228-6. [DOI] [PubMed] [Google Scholar]
- Streicher S. L., Gurney E. G., Valentine R. C. The nitrogen fixation genes. Nature. 1972 Oct 27;239(5374):495–499. doi: 10.1038/239495a0. [DOI] [PubMed] [Google Scholar]
- Streicher S. L., Shanmugam K. T., Ausubel F., Morandi C., Goldberg R. B. Regulation of nitrogen fixation in Klebsiella pneumoniae: evidence for a role of glutamine synthetase as a regulator of nitrogenase synthesis. J Bacteriol. 1974 Nov;120(2):815–821. doi: 10.1128/jb.120.2.815-821.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Streicher S., Gurney E., Valentine R. C. Transduction of the nitrogen-fixation genes in Klebsiella pneumoniae. Proc Natl Acad Sci U S A. 1971 Jun;68(6):1174–1177. doi: 10.1073/pnas.68.6.1174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tubb R. S. Glutamine synthetase and ammonium regulation of nitrogenase synthesis in Klebsiella. Nature. 1974 Oct 11;251(5475):481–485. doi: 10.1038/251481a0. [DOI] [PubMed] [Google Scholar]
- Tubb R. S., Postgate J. R. Control of nitrogenase synthesis in Klebsiella pneumoniae. J Gen Microbiol. 1973 Nov;79(1):103–117. doi: 10.1099/00221287-79-1-103. [DOI] [PubMed] [Google Scholar]
- Tyler B., Deleo A. B., Magasanik B. Activation of transcription of hut DNA by glutamine synthetase. Proc Natl Acad Sci U S A. 1974 Jan;71(1):225–229. doi: 10.1073/pnas.71.1.225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolf B., Newman A., Glaser D. A. On the origin and direction of replication of the Escherichia coli K12 chromosome. J Mol Biol. 1968 Mar 28;32(3):611–629. doi: 10.1016/0022-2836(68)90346-x. [DOI] [PubMed] [Google Scholar]
- Yoch D. C., Pengra R. M. Effect of amino acids on the nitrogenase system of Klebsiella pneumoniae. J Bacteriol. 1966 Sep;92(3):618–622. doi: 10.1128/jb.92.3.618-622.1966. [DOI] [PMC free article] [PubMed] [Google Scholar]


