Abstract
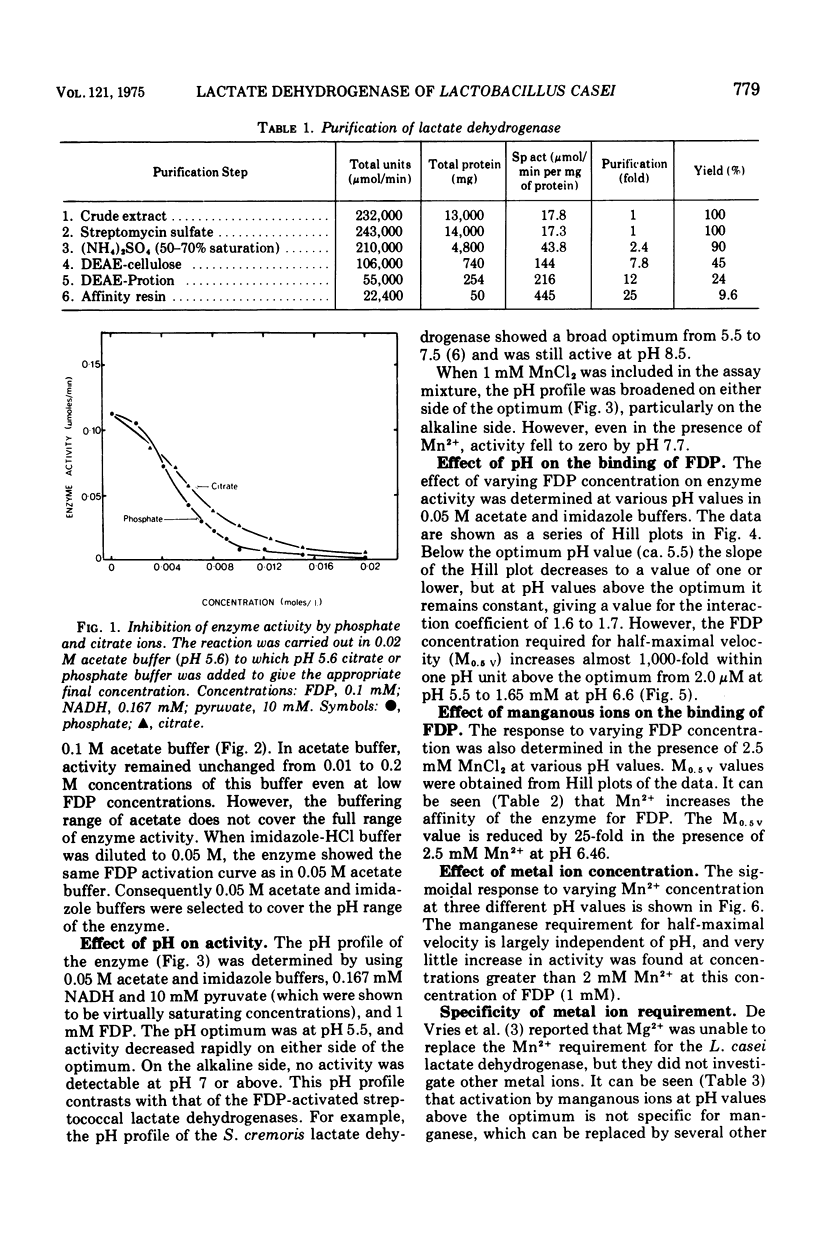
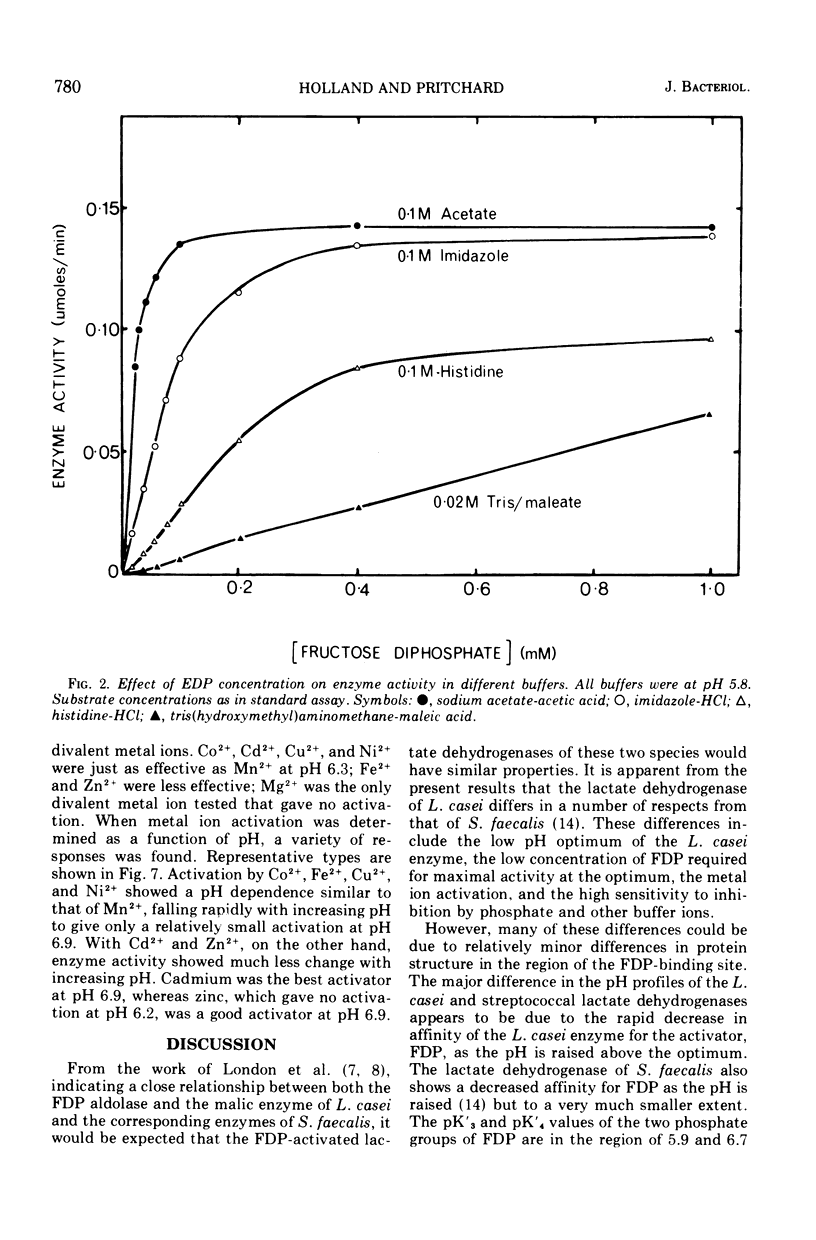
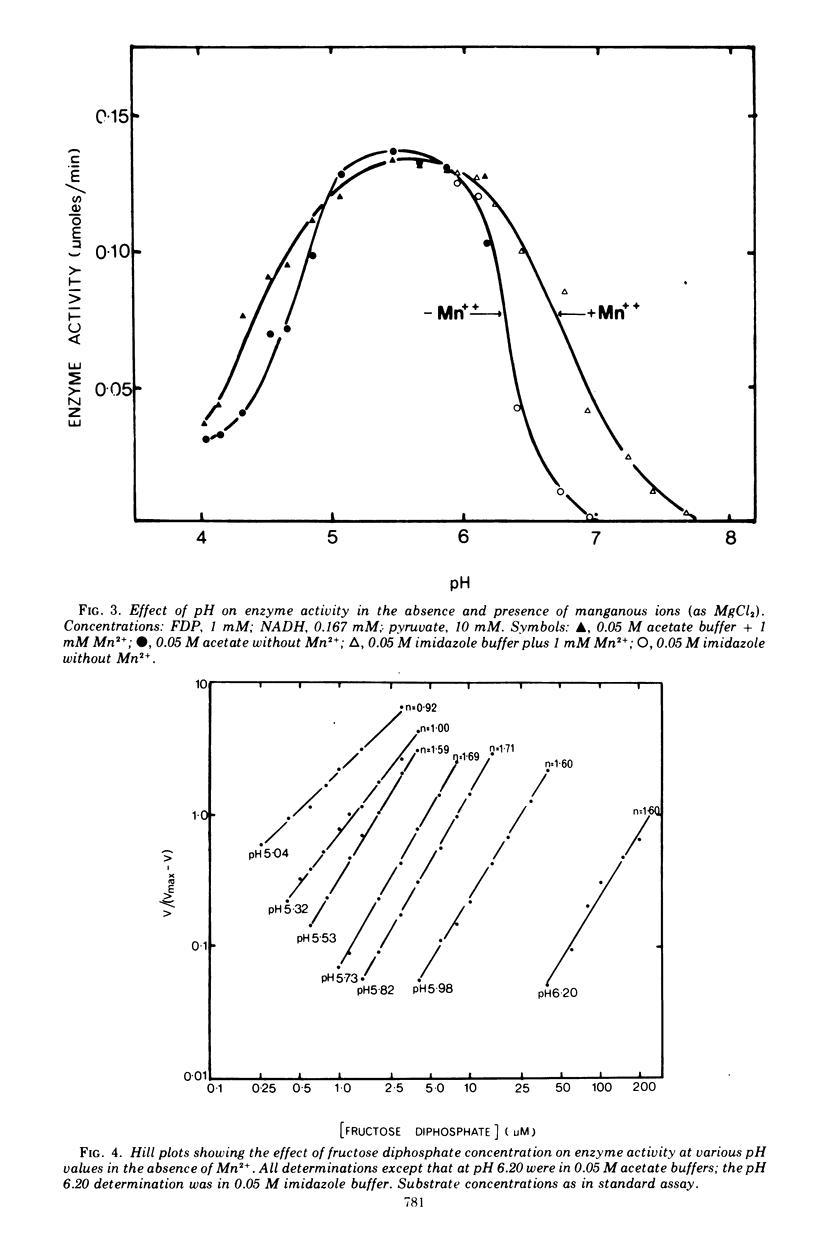
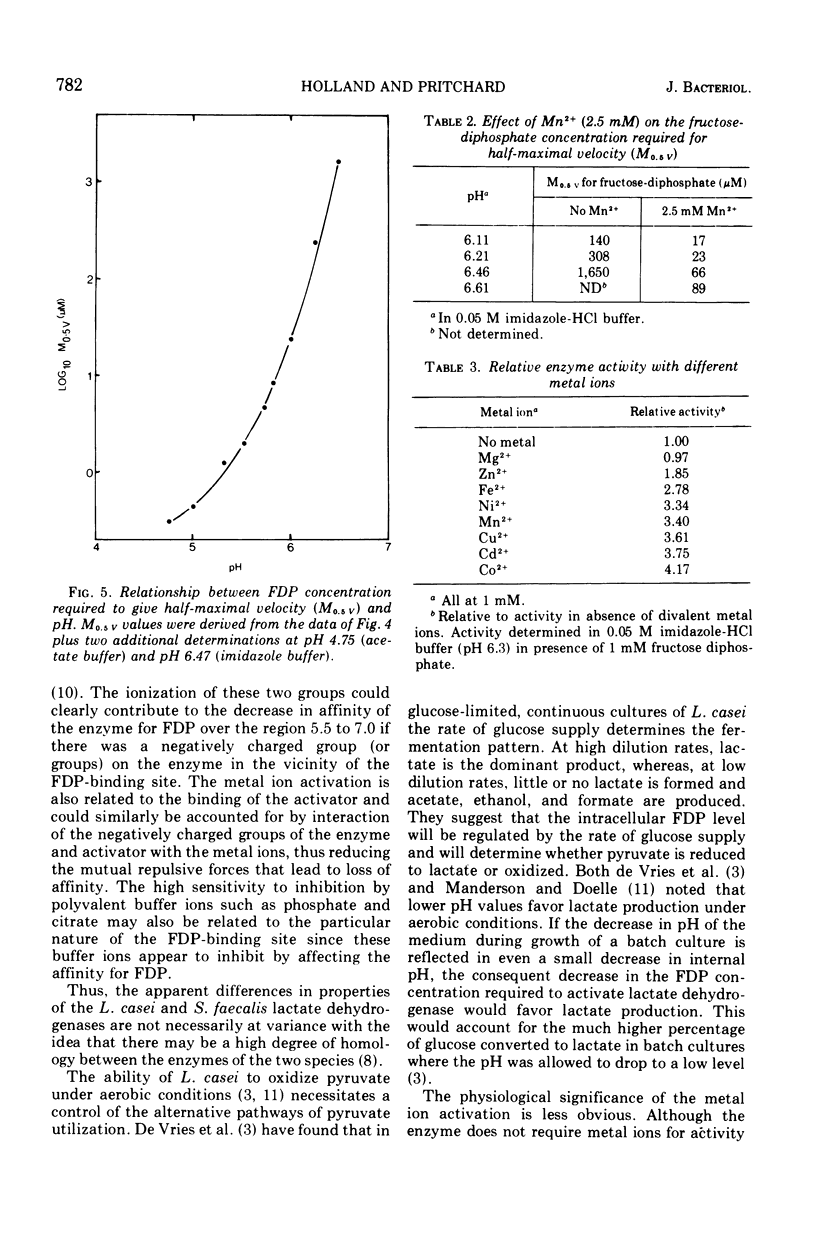
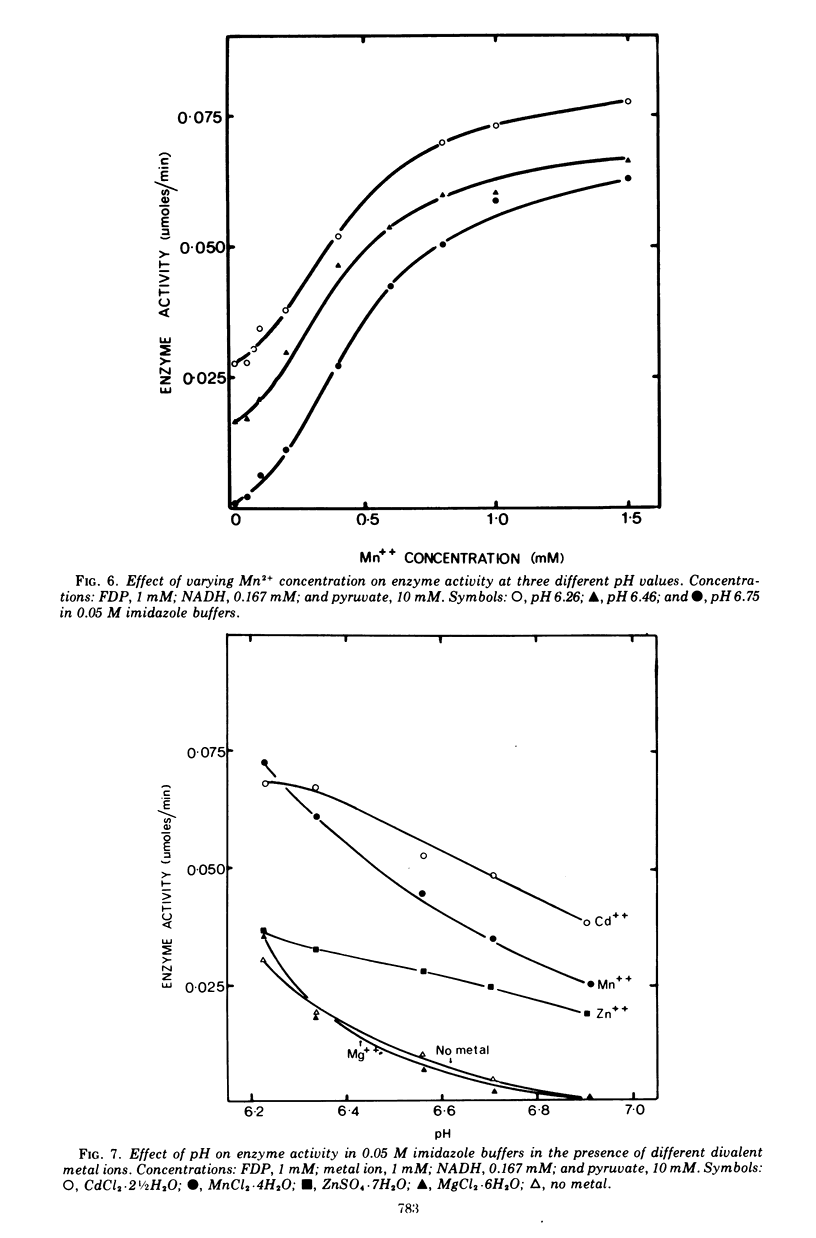
The lactate dehydrogenase of Lactobacillus casei, like that of streptococci, requires fructose-1,6-diphosphate (FDP) for activity. The L. casei enzyme has a much more acidic pH optimum (pH 5.5) than the streptococcal lactate dehydrogenases. This is apparently due to a marked decrease in the affinity of the enzyme for the activator with increasing pH above 5.5; the concentration of FDP required for half-maximal velocity increase nearly 1,000-fold from 0.002 mM at pH 5.5 to 1.65 mM at 6.6. Manganous ions increase the pH range of activity particularly on the alkaline side of the optimum by increasing the affinity for FDP. This pH dependent metal ion activation is not specific for Mn2+. Other divalent metals, Co2+, Cu2+, Cd2+, Ni2+, Fe2+, Fe2+, and Zn2+ but not Mg2+, will effectively substitute for Mn2+, but the pH dependence of the activation differs with the metal ion used. The enzyme is inhibited by a number of commonly used buffering ions, particularly phosphate, citrate, and tris (hydroxymethyl) aminomethane-maleate buffers, even at low buffer concentrations (0.02 M). These buffers inhibit by affecting the binding of FDP.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Cuatrecasas P. Protein purification by affinity chromatography. Derivatizations of agarose and polyacrylamide beads. J Biol Chem. 1970 Jun;245(12):3059–3065. [PubMed] [Google Scholar]
- Gasser F. Electrophoretic characterization of lactic dehydrogenases in the genus Lactobacillus. J Gen Microbiol. 1970 Aug;62(2):223–239. doi: 10.1099/00221287-62-2-223. [DOI] [PubMed] [Google Scholar]
- Jonas H. A., Anders R. F., Jago G. R. Factors affecting the activity of the lactate dehydrognease of Streptococcus cremoris. J Bacteriol. 1972 Aug;111(2):397–403. doi: 10.1128/jb.111.2.397-403.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- London J., Kline K. Aldolase of lactic acid bacteria: a case history in the use of an enzyme as an evolutionary marker. Bacteriol Rev. 1973 Dec;37(4):453–478. doi: 10.1128/br.37.4.453-478.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- London J., Meyer E. Y., Kulczyk S. R. Detection of relationships between Streptococcus faecalis and Lactobacillus casei by immunological studies with two forms of malic enzyme. J Bacteriol. 1971 Oct;108(1):196–201. doi: 10.1128/jb.108.1.196-201.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manderson G. J., Doelle H. W. The effect of oxygen and pH on the glucose metabolism of Lactobacillus casei var. rhamnosus ATCC 7469. Antonie Van Leeuwenhoek. 1972;38(2):223–240. doi: 10.1007/BF02328095. [DOI] [PubMed] [Google Scholar]
- WOLIN M. J. FRUCTOSE-1,6-DIPHOSPHATE REQUIREMENT OF STREPTOCOCCAL LACTIC DEHYDROGENASES. Science. 1964 Nov 6;146(3645):775–777. doi: 10.1126/science.146.3645.775. [DOI] [PubMed] [Google Scholar]
- Wittenberger C. L., Angelo N. Purificationa and properties of a fructose-1,6-diphosphate-activated lactate dehydrogenase from Streptococcus faecalis. J Bacteriol. 1970 Mar;101(3):717–724. doi: 10.1128/jb.101.3.717-724.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Vries W., Gerbrandy S. J., Stouthamer A. H. Carbohydrate metabolism in Bifidobacterium bifidum. Biochim Biophys Acta. 1967 Apr 25;136(3):415–425. doi: 10.1016/0304-4165(67)90001-3. [DOI] [PubMed] [Google Scholar]
- de Vries W., Kapteijn W. M., van der Beek E. G., Stouthamer A. H. Molar growth yields and fermentation balances of Lactobacillus casei L3 in batch cultures and in continuous cultures. J Gen Microbiol. 1970 Nov;63(3):333–345. doi: 10.1099/00221287-63-3-333. [DOI] [PubMed] [Google Scholar]


