Abstract
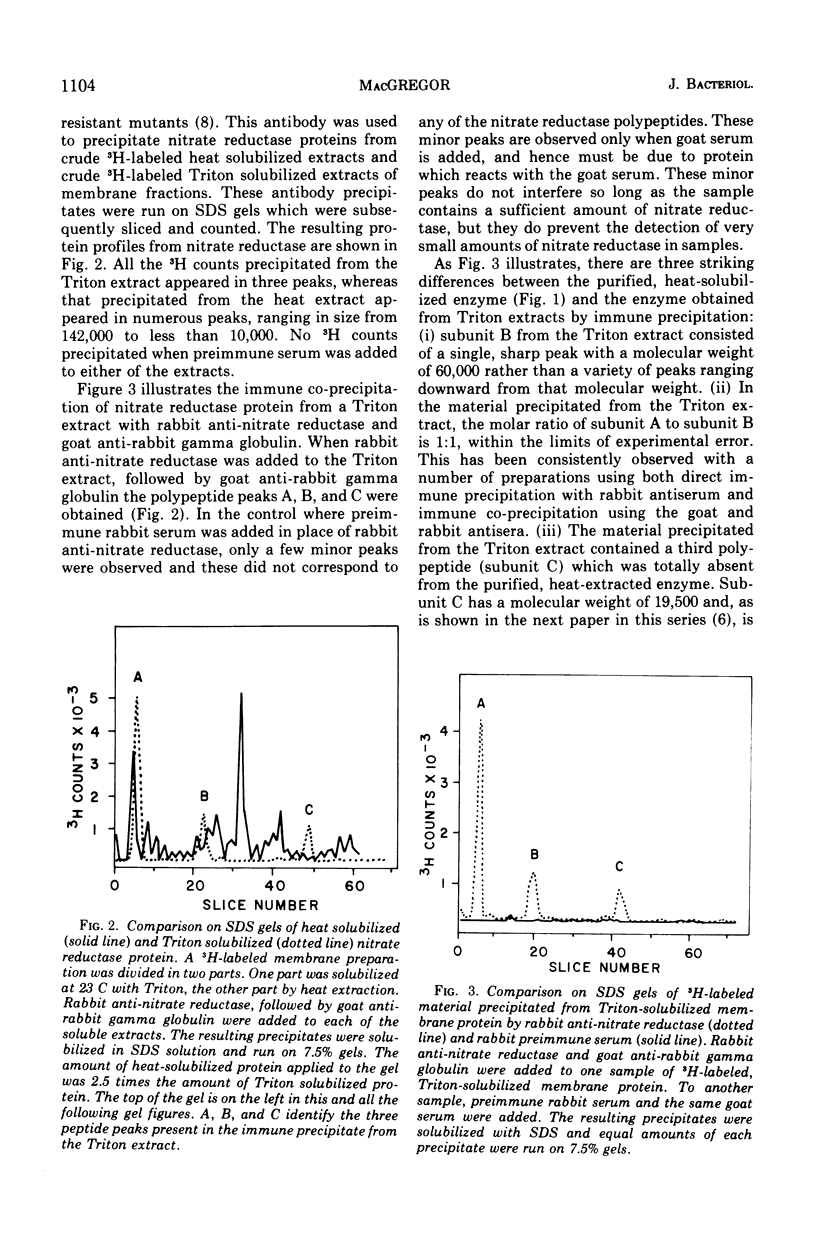
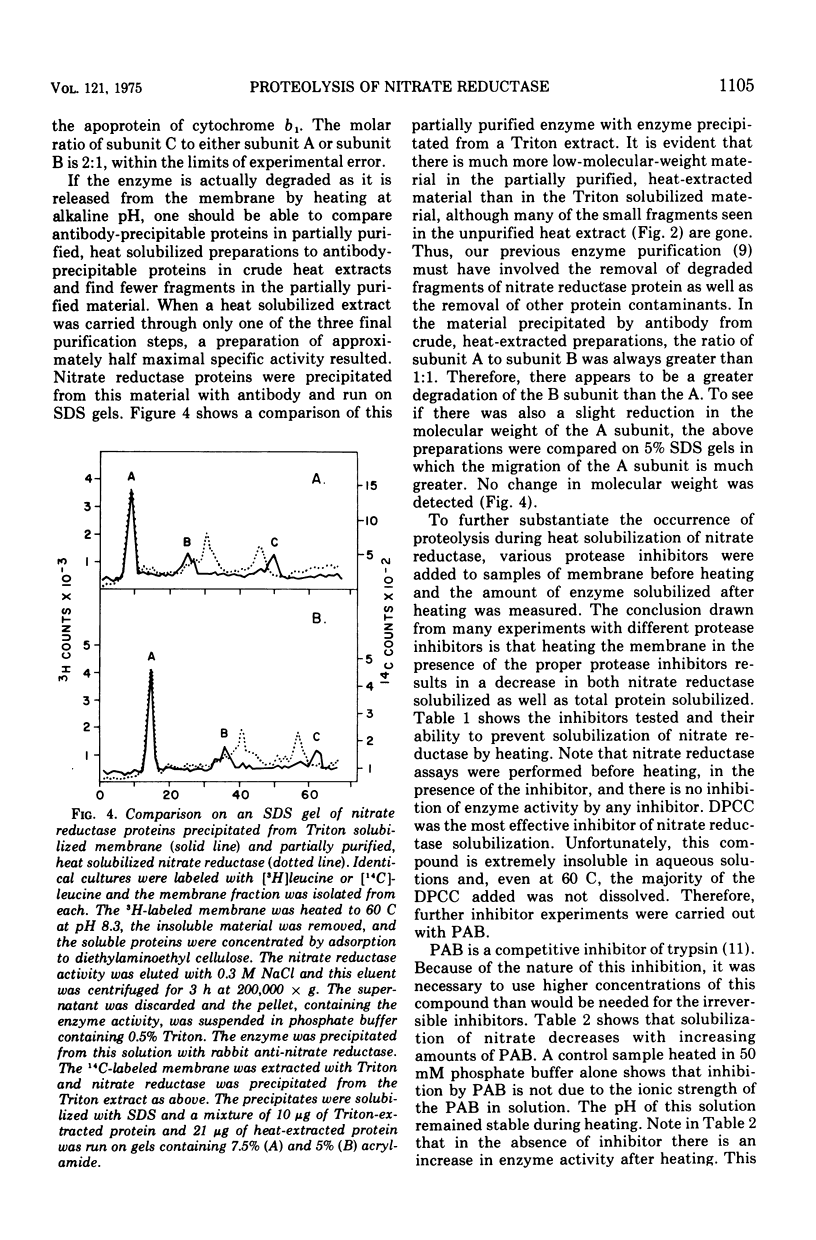
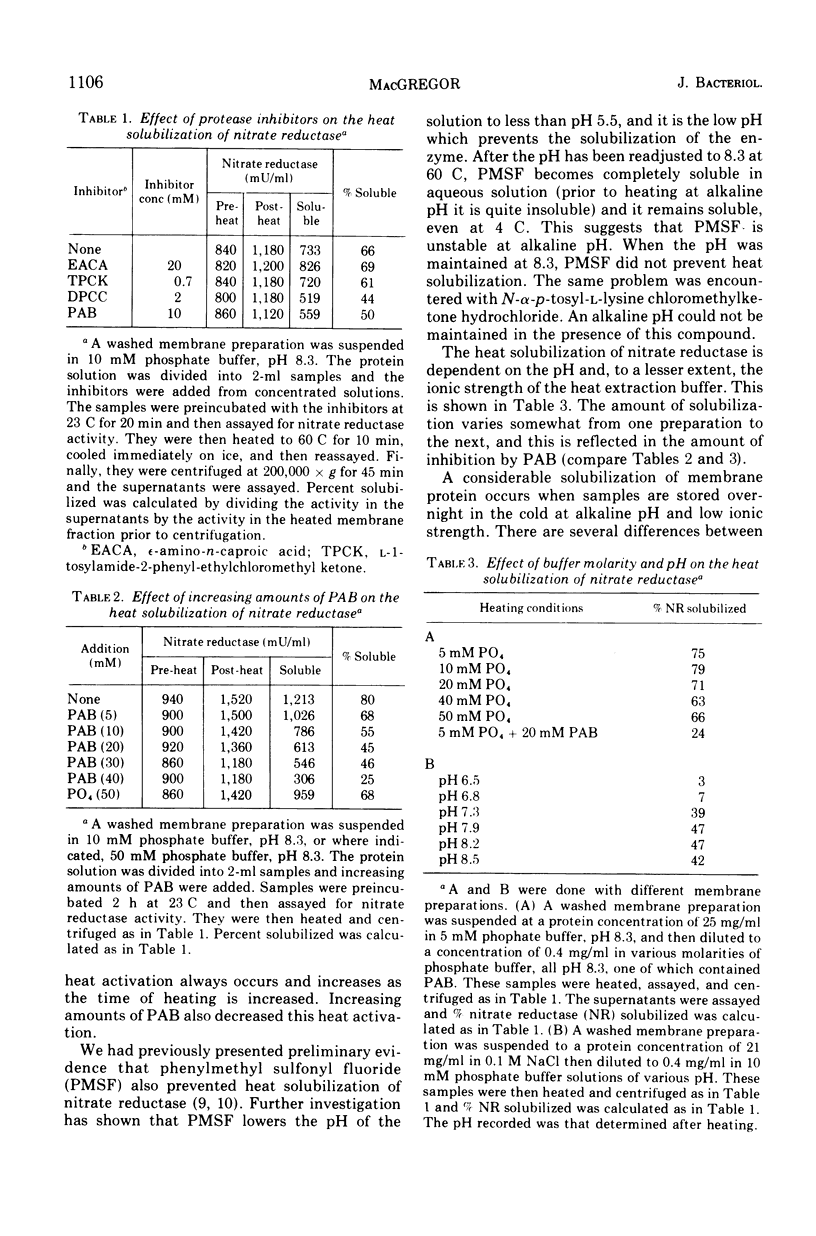
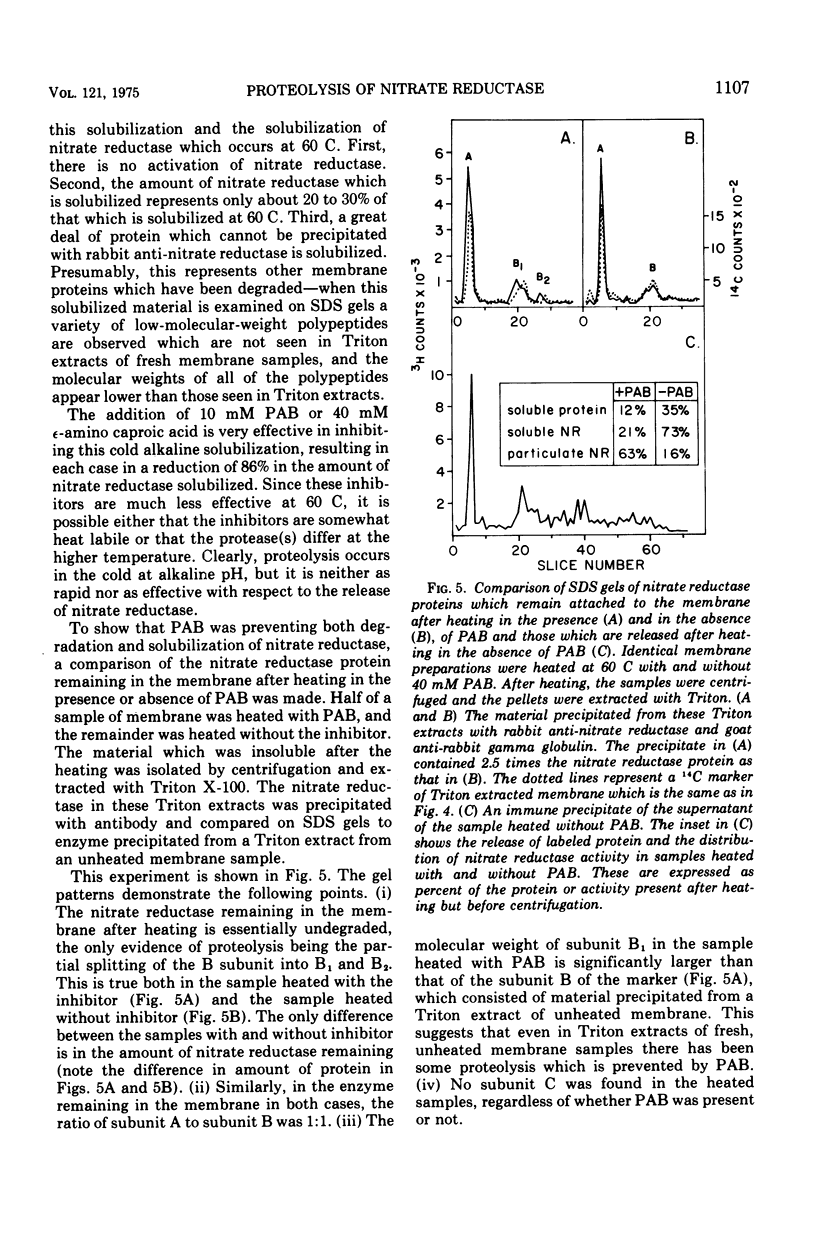
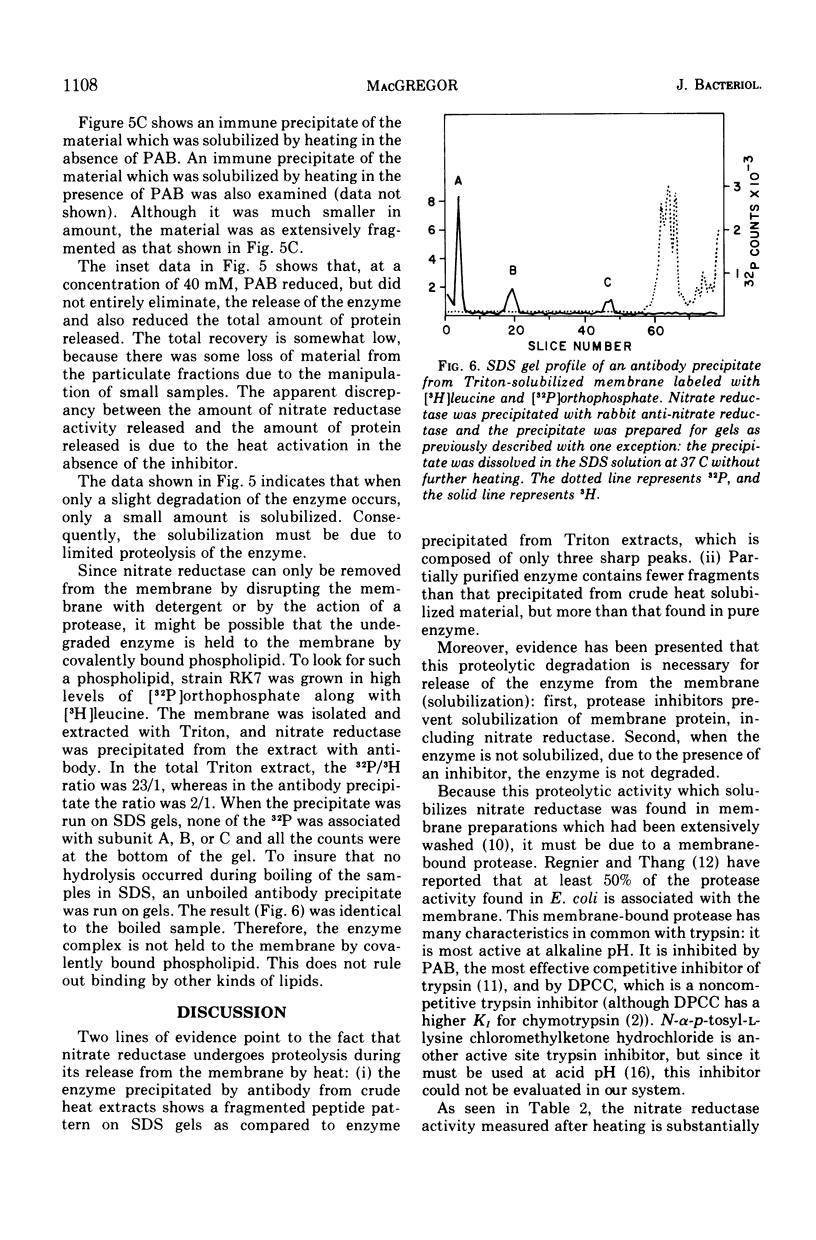
Nitrate reductase extracted from the membrane of Escherichia coli by alkaline heat treatment was purified to homogeneity and used to prepare specific antibody. Nitrate reductase, precipitated by this antibody from Triton extracts of the membrane, contained a third subunit not present in the purified enzyme used to prepare the antibody. Nitrate reductase precipitated by antibody from alkaline heat extracts was composed of peptide fragments of various sizes. These fragments were produced by a membrane-bound protease which was activated by alkaline pH and heat. It is the action of this protease that releases the enzyme from the membrane, as shown by the observations that protease inhibitors decreased the amount of solubilization of the enzyme, and the enzyme remaining in the membrane after heating showed much less proteolytic cleavage than that which was released.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Baron C., Abrams A. Isolation of a bacterial membrane protein, nectin, essential for the attachment of adenosine triphosphatase. J Biol Chem. 1971 Mar 10;246(5):1542–1544. [PubMed] [Google Scholar]
- JACOBS E. E., SANADI D. R. The reversible removal of cytochrome c from mitochondria. J Biol Chem. 1960 Feb;235:531–534. [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Lester R. L., DeMoss J. A. Effects of molybdate and selenite on formate and nitrate metabolism in Escherichia coli. J Bacteriol. 1971 Mar;105(3):1006–1014. doi: 10.1128/jb.105.3.1006-1014.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MARES-GUIA M., SHAW E. STUDIES ON THE ACTIVE CENTER OF TRYPSIN. THE BINDING OF AMIDINES AND GUANIDINES AS MODELS OF THE SUBSTRATE SIDE CHAIN. J Biol Chem. 1965 Apr;240:1579–1585. [PubMed] [Google Scholar]
- MacGregor C. H. Anaerobic cytochrome b1 in Escherichia coli: association with and regulation of nitrate reductase. J Bacteriol. 1975 Mar;121(3):1111–1116. doi: 10.1128/jb.121.3.1111-1116.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacGregor C. H., Schnaitman C. A. Alterations in the cytoplasmic membrane proteins of various chlorate-resistant mutants of Escherichia coli. J Bacteriol. 1971 Oct;108(1):564–570. doi: 10.1128/jb.108.1.564-570.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacGregor C. H., Schnaitman C. A., Normansell D. E. Purification and properties of nitrate reductase from Escherichia coli K12. J Biol Chem. 1974 Aug 25;249(16):5321–5327. [PubMed] [Google Scholar]
- MacGregor C. H., Schnaitman C. A. Reconstitution of nitrate reductase activity and formation of membrane particles from cytoplasmic extracts of chlorate-resistant mutants of Escherichia coli. J Bacteriol. 1973 Jun;114(3):1164–1176. doi: 10.1128/jb.114.3.1164-1176.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Régnier P., Thang M. N. Membrane associated proteases in E. coli. FEBS Lett. 1973 Oct 1;36(1):31–33. doi: 10.1016/0014-5793(73)80330-8. [DOI] [PubMed] [Google Scholar]
- Sandermann H., Jr, Strominger J. L. C 55 -isoprenoid alcohol phosphokinase: an extremely hydrophobic protein from the bacterial membrane. Proc Natl Acad Sci U S A. 1971 Oct;68(10):2441–2443. doi: 10.1073/pnas.68.10.2441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schnaitman C. A. Protein composition of the cell wall and cytoplasmic membrane of Escherichia coli. J Bacteriol. 1970 Nov;104(2):890–901. doi: 10.1128/jb.104.2.890-901.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schnaitman C. A. Solubilization of the cytoplasmic membrane of Escherichia coli by Triton X-100. J Bacteriol. 1971 Oct;108(1):545–552. doi: 10.1128/jb.108.1.545-552.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Showe M. K., DeMoss J. A. Localization and regulation of synthesis of nitrate reductase in Escherichia coli. J Bacteriol. 1968 Apr;95(4):1305–1313. doi: 10.1128/jb.95.4.1305-1313.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spatz L., Strittmatter P. A form of cytochrome b5 that contains an additional hydrophobic sequence of 40 amino acid residues. Proc Natl Acad Sci U S A. 1971 May;68(5):1042–1046. doi: 10.1073/pnas.68.5.1042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strittmatter P., Rogers M. J., Spatz L. The binding of cytochrome b 5 to liver microsomes. J Biol Chem. 1972 Nov 25;247(22):7188–7194. [PubMed] [Google Scholar]
- Swank R. T., Munkres K. D. Molecular weight analysis of oligopeptides by electrophoresis in polyacrylamide gel with sodium dodecyl sulfate. Anal Biochem. 1971 Feb;39(2):462–477. doi: 10.1016/0003-2697(71)90436-2. [DOI] [PubMed] [Google Scholar]


